Abstract
Introduction: Stratification of multiple myeloma patient responses by reductions in monoclonal component is based upon reliable, historic outcome data. With the introduction of novel agents, a greater number of patients are achieving hitherto unthought-of responses, with many expecting to meet the requirement for VGPR or greater. Multiple factors can impact the quantification and assessment of response in patients achieving deep responses including the influence of IgG recycling, accuracy of quantitation against an increasing polyclonal background and the presence of monoclonal immunotherapies. Alternative strategies for monoclonal protein measurement include heavy+light chain (HLC) immunoassays. Here for the first time we compare the consistency of response assignment between the two methods and evaluate the clinical impact of discordance.
Methods: Sera from 509 newly diagnosed intact immunoglobulin multiple myeloma (IIMM) patients enrolled onto IFM-2009 trial were available (median age was 59 (range: 33-66) years, follow-up 30 (3-56) months). Two patients arms were treated with RVDx3+ASCT+RVDx2 or RVDx8, followed by 12 months Lenalidomide maintenance. Responses were assigned at the end of consolidation therapy according to IMWG criteria using changes in monoclonal protein concentrations measured by either SPE or dHLC (clonal - non clonal). Complete response (CR) was assigned by either method by the absence of monoclonal protein on IFE or a normal HLC ratio (HLCr), respectively, and <5% BM plasma cells. HLC-pair suppression was defined as levels of the non-clonal HLC (e.g. IgGλ in an IgGκ patient) below the reference range (IgGκ <3.84g/L; IgGλ <1.91g/L; IgAκ <0.57g/L; IgAλ <0.44g/L). Minimal residual disease (MRD) was assessed by 7-color flow cytometry at the end of consolidation therapy.
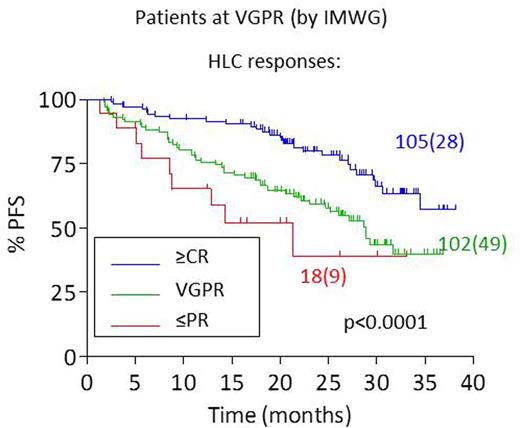
Results: IFE and HLCr concordantly identified clonality in 99% patients. In 463 patients followed until the end of consolidation we found moderate agreement in response assignment between the two tests. HLC gave similar response assignments to electrophoretic tests in patients with poor response (≤PR, 76/96; 79%) or complete response (≥CR, 130/142; 92%). However in patients achieving VGPR there was considerable discordance between methods for response assignment (102/225; 45%). Having identified discordant results for VGPR we sought to assess the clinical accuracy of the responses assigned within this group. Of the 225 patients with standard VGPR, HLC response assignment varied (PR (18/225), VGPR (102/225) and ≥CR (105/225)). Median PFS for patients achieving standard VGPR was 34.5 months; responses by HLC further described these patients into those with poorer PFS (PR, median PFS=21.3 months (95%CI: 9.0-33.7)); VGPR (PFS=28.9 months (95%CI: 25.1-32.6)), and those with improved outcomes (CR, median PFS not reached); p<0.0001 (Figure 1). This suggested that the response assigned by electrophoresis could be refined by HLC analysis. By contrast, responses assigned by electrophoretic methods offered no added clinical value in patients achieving VGPR (log-rank: p=0.724) or ≥CR (log-rank: p=0.402) by HLC assessment. Furthermore, an abnormal HLCr post-consolidation had a greater concordance with MRD+ assessment (78% positive) compared to IFE (51% positive); whereas both a normal HLCr and negative IFE displayed similar agreement with MRD- assessment (88% and 94% negative, respectively).
Finally we assessed the clinical impact of recovering polyclonal immunoglobulin levels after consolidation therapy. In 142 patients with complete responses, HLC-pair suppression (n=15, median PFS=35.1 months (95%CI: 20.9-49.2) and severe suppression (n=7, median PFS=22.9 months (95%CI: 16.6-29.2)) were associated with poorer outcomes (p=0.060 and p=0.004, respectively).
Conclusions: In patients achieving a poor or exceptional response there was good agreement between HLC and standard response assignment. For patients achieving a VGPR we suggest that HLC response assignment may be beneficial, therefore overall the assessment could be used to augment or replace electrophoresis. The prognostic significance of HLC responses seems to be partly dependent in the patient's ability to recover their immune system, as determined by normalisation of non-clonal HLC levels.
Michallet:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Astellas Pharma: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria. Attal:sanofi: Consultancy; janssen: Consultancy, Research Funding; amgen: Consultancy, Research Funding; celgene: Consultancy, Research Funding. Moreau:Novartis: Honoraria; Celgene: Honoraria; Takeda: Honoraria; Janssen: Honoraria, Speakers Bureau; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria. Avet-Loiseau:janssen: Consultancy; sanofi: Consultancy; amgen: Consultancy; celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal