Abstract
Background: Ibrutinib is an irreversible inhibitor of Bruton's tyrosine kinase (BTK) that induces durable remissions in chronic lymphocytic leukemia (CLL). In other systems, ibrutinib has also been shown to have an immune modulating effect on T-cells through inhibition of interleukin-associated tyrosine kinase (ITK). Our group and then others have reported that ibrutinib enhances antibacterial and antitumor T cell immune response in murine models. ITK has been demonstrated to be involved in TCR-induced up-regulation of FAS Ligand (FAS-L) and activation induced cell death (AICD) of T cells. In murine models, ITK deficiency or inhibition by specific kinase inhibitors lead to impaired AICD and a much greater persistence and accumulation of activated T cells. We therefore postulate that ibrutinib, by its capability to irreversibly inhibit ITK, may have similar effect on recusing activated T cells from AICD. To date, a comprehensive analysis of the effects on cellular immunity in humans treated with ibrutinib, or more selective BTK inhibitors has not been reported.
Methods: PBMCs from previously treated CLL patients enrolled on clinical trials (NCT01589302 and NCT02029443) of ibrutinib (a BTK and ITK inhibitor) and acalabrutinib (ACP-196, a selective BTK inhibitor) were collected at baseline and during treatment at the completion of 2 months( cycle 3) and 5 months (cycle 6). Patients with persistent lymphocytosis post treatment were chosen to allow comparable tumor burden before and after therapy. T cell immune function and the immunosuppressive capacity of CLL cells were evaluated with detailed immunologic and functional studies. Mechanistic studies examining AICD with ibrutinib and acalabrutinib treatment were performed.
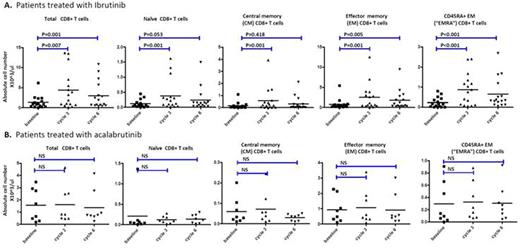
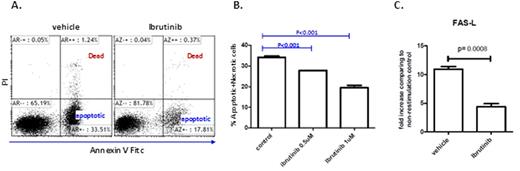
Results: A total of 25 patients treated with ibrutinib (n=17) and acalabrutinib (n=8) were studied. Ibrutinib, but not acalabrtinib treatment promoted a significant increase (3-5 fold) in absolute CD4(P<0.01) and CD8(p=0.001) T cell numbers in CLL patients that was more prominent in effector/effector memory subsets and less in naïve and central memory subsets (Figure 1). While the number of regulatory T cells (Treg cells) remained unchanged, the ratio of Treg cells to CD4 T cells was reduced by approximately two fold (p<0.001) after ibrutinib treatment. Detailed ex vivo studies demonstrated significantly diminished activation induced cell death of activated T-cells when pre-treated with ibrutinib, but not acalabrutinib (Figure 2). Treatment with ibrutinib or acalabrutinib significantly reduced T cell PD-1 (p<0.01 for CD8 T cells, p<0.05 for CD4 T cells and CTLA-4 (p< 0.05 for CD8 T cells, p<0.001 for CD4 T cells) expression, most prominent in central memory T cell compartment. Ibrutinib and acalabrutinib both significantly decreased CD200 (p<0.001) and BTLA (p<0.001) surface expression as well as IL-10 production by CLL cells after prolonged "B10-Pro" conditioning (p<0.001 for ibrutinib, p<0.05 for acalabrutinib).
Conclusions:These findings collectively differentiate ibrutinib as a therapeutic that can both expand activated T-cells and also ameliorate T-cell exhaustion thorough reversal of CLL tumor cell mediated immune suppression. While acalabrutinib reverses CLL cell mediated immune suppression, it lacks ability to expand activated T-cells. Collectively, these data provide support for dual BTK/ITK inhibitor therapeutics when applied to enhance the therapeutic efficacy of adoptive immunotherapy (e.g.chimeric antigen receptor T cells) or tumor vaccines for CLL and potentially other types of hematologic and solid cancers.
Ibrutinib but not acalabrutinib treatment of CLL patients increases total T cell numbers: (A) Absolute numbers of CD8 T cells before and during ibrutinib treatment (n=18). (B) Absolute numbers of CD8 T cells before and during acalabrutinib treatment (n=8). Each cycle is 4 weeks. "Cycle 3" day 1 indicates 8 weeks into treatment, and "cycle 6" day 1 indicates 20 weeks into treatment.
Ibrutinib but not acalabrutinib treatment of CLL patients increases total T cell numbers: (A) Absolute numbers of CD8 T cells before and during ibrutinib treatment (n=18). (B) Absolute numbers of CD8 T cells before and during acalabrutinib treatment (n=8). Each cycle is 4 weeks. "Cycle 3" day 1 indicates 8 weeks into treatment, and "cycle 6" day 1 indicates 20 weeks into treatment.
Ibrutinib treatment of human T cells protects against activation induced cell death and reduces FAS-L mRNA expression in a dose dependent manor. T cells were isolated from healthy donors blood samples, stimulated in vitro with CD3/CD28 for 3 days, rested for 11 days, then were restimulated with CD3 for 6 hours (as in A. and B.) or 3 hours (as in C.) in the presence of IL2 to induce AICD in the presence or absence of ibrutinib.
Ibrutinib treatment of human T cells protects against activation induced cell death and reduces FAS-L mRNA expression in a dose dependent manor. T cells were isolated from healthy donors blood samples, stimulated in vitro with CD3/CD28 for 3 days, rested for 11 days, then were restimulated with CD3 for 6 hours (as in A. and B.) or 3 hours (as in C.) in the presence of IL2 to induce AICD in the presence or absence of ibrutinib.
Jones:AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding. Andritsos:Hairy Cell Leukemia Foundation: Research Funding. Awan:Novartis Oncology: Consultancy; Innate Pharma: Research Funding; Pharmacyclics: Consultancy. Fraietta:Novartis: Patents & Royalties: Novartis, Research Funding. June:Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; Tmunity: Equity Ownership, Other: Founder, stockholder ; University of Pennsylvania: Patents & Royalties; Immune Design: Consultancy, Equity Ownership; Pfizer: Honoraria; Johnson & Johnson: Research Funding; Celldex: Consultancy, Equity Ownership. Maus:Novartis: Patents & Royalties: inventor on patents related to ibrutinib use in T cell therapies. Woyach:Karyopharm: Research Funding; Morphosys: Research Funding; Acerta: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal