Abstract
Background: The oral selective BCL-2 inhibitor, venetoclax, is FDA-approved for the treatment of Chronic Lymphocytic Leukemia (CLL) patients with 17p deletion [del(17p)] who have received at least one prior therapy.Multiple clinical trials investigating its activity in patients with relapsed or refractory CLL are ongoing, and longer term follow-up is now available since the original publications of the initial Phase 1 and 2 trials. In order to define the response rate and durability of response across a broader population than studied in any individual trial, we performed a pooled analysis of current data available from four ongoing studies.
Methods: Data were analyzed from the following venetoclax trials - NCT01328626 (M12-175 study): phase 1, venetoclax monotherapy in patients with relapsed or refractory CLL (n=116, data as of 04APR2016,); NCT02141282 (M14-032 study): phase 2, venetoclax monotherapy for CLL patients who have progressed on ibrutinib or idelalisib (n=64, data as of 28JUN2016, and excludes safety expansion patients whose long term data are not available); NCT01889186 (M13-982 study): phase 2, venetoclax monotherapy in relapsed or refractory CLL patients with del(17p) (n=158, data as of 29MAR2016); NCT01682616 (M13-365 study): phase 1b, venetoclax plus rituximab in relapsed or refractory CLL (n=49; data as of 04MAR2016).
Bone marrow minimal residual disease (MRD) status was determined using data from the M13-982 and M13-365 studies (n=207). MRD data for the M14-032 study is not yet available and MRD assessment was not systematically performed for the M12-175 study. Del(17p) status was assessed using fluorescence in situ hybridization and TP53 mutation status was determined by next generation sequencing. Descriptive statistics (median, range, proportion) were calculated for demographics, baseline characteristics, and overall response. All post-baseline data were included to determine the overall response and did not account for differences in the follow-up times between studies. Kaplan-Meier methodology was used to calculate 24-month estimates and associated 95% confidence interval (CI) for duration of response (DOR) and progression free survival (PFS).
Results: A total of 387 patients were included in this analysis [median age: 66 years (range: 29 - 88)]. Bulky nodes (≥5 cm) were present in 194/386 (50%) patients and del(17p) was confirmed in 211/356 (59%) patients with available data; 207/387 (53%) patients had ≥3 prior therapies. Venetoclax doses ranged from 150 mg/day to 1200 mg/day; 299/387 (77%) patients received the recommended phase 2 dose (RPTD) of 400 mg/day. Across all four studies, the median duration on venetoclax was 15.3 (range: 0 - 51.8) months and the median time on study was 16.6 months (range: 0 - 51.8). 54% of the patients continue on venetoclax; 46% have discontinued due to: progressive disease (30%), adverse events (9%), stem cell transplant (3%), withdrew consent (3%), investigator request, lost to follow-up, non-compliance (n=1, 0.25% each).
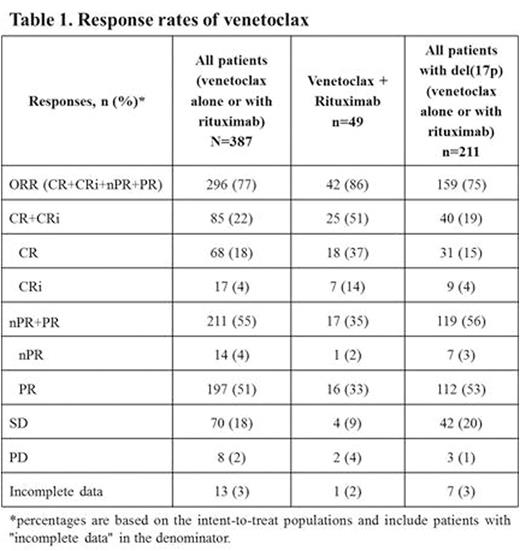
The overall response rate (ORR) in all patients was 77% (Table 1). The CR/CRi (complete remission/complete remission with incomplete marrow recovery) rate was 22% and the number increased over time on venetoclax [median time to CR/CRi = 8.3 (range: 2.6 - 28.6) months]; MRD-negativity in the marrow was achieved by 21% (44/207) of patients, including 12% (19/157) of those with del(17p).
The impact of clinical response, marrow MRD negativity, and del(17p) or TP53 status on DOR and PFS is summarized in Table 2. For all four studies combined, the median DOR was 29.2 months (95% CI=25.1, 40.1) and the median PFS was 27.9 months (95% CI=24.9, 30.5). The 24-month estimate for DOR in all patients was 64.3% (95% CI=55.5, 71.8) and that for PFS was 56.9% (95% CI=50.3, 63). Patients who achieved marrow MRD negativity had the highest 24-month DOR estimate of 96.6% (95% CI= 77.9, 99.5), followed by those with CR+CRi [89.8% (95% CI=73.5, 96.3)] and those without del(17p) or TP53 mutation [74.6 (95% CI=62.3, 83.4)]. Similar trends were seen for the 24-month PFS estimates.
Conclusions: Pooled data from the four studies show that venetoclax induces deep (22% CR/CRi and 21% marrow MRD negativity rates) and durable responses in patients with relapsed/refractory CLL. DOR and PFS were the most favorable in patients who achieved marrow MRD negativity and CR/CRi and those without del(17p) or TP53 mutation.
Roberts:Servier: Research Funding; Janssen: Research Funding; Genentech: Patents & Royalties: Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone payments related to venetoclax; AbbVie: Research Funding; Genentech: Research Funding. Seymour:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Eichhorst:Abbvie: Consultancy; Mundipharma: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau. Stilgenbauer:Janssen: Consultancy, Honoraria, Other: Travel grants , Research Funding; GSK: Consultancy, Honoraria, Other: Travel grants , Research Funding; Novartis: Consultancy, Honoraria, Other: Travel grants , Research Funding; Sanofi: Consultancy, Honoraria, Other: Travel grants , Research Funding; Amgen: Consultancy, Honoraria, Other: Travel grants, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel grants, Research Funding; Celgene: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genentech: Consultancy, Honoraria, Other: Travel grants , Research Funding; Gilead: Consultancy, Honoraria, Other: Travel grants , Research Funding; Boehringer Ingelheim: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genzyme: Consultancy, Honoraria, Other: Travel grants , Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel grants , Research Funding; Mundipharma: Consultancy, Honoraria, Other: Travel grants , Research Funding; Hoffmann-La Roche: Consultancy, Honoraria, Other: Travel grants , Research Funding. Davids:Pharmacyclics: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Gilead: Honoraria; Abbvie: Consultancy, Honoraria; TG Therapeutics: Honoraria, Research Funding; Infinity: Honoraria, Research Funding. Gerecitano:AbbVie: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Samus: Consultancy, Honoraria; Gilead: Consultancy, Honoraria. Popovic:AbbVie Inc.: Employment, Other: may own stock. Chyla:AbbVie Inc.: Employment, Other: may own stock. Zhu:AbbVie Inc.: Employment, Other: may own stock. Verdugo:AbbVie: Employment, Other: may own stock. Potluri:AbbVie Inc.: Employment, Other: may own stock. Lash:AbbVie Inc.: Employment, Other: may own stock. Kim:AbbVie Inc.: Employment, Other: may own stock. Hallek:Celgene: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; F. Hoffmann-LaRoche: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Mundipharma: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal