Abstract
Background: Cirmtuzumab is a first-in-class humanized mAb specific for ROR1, an oncoembryonic antigen found on CLL and cancer stem cells of various cancers, but not on normal post-partum tissues. Work has defined that ROR1 serves as a receptor for Wnt5a, which induces non-canonical Wnt-signaling that promotes planar-cell-polarity, migration, stem-cell renewal, and proliferation. Recent studies demonstrated that Wnt5a binds to ROR1 and induces activation of RhoA and Rac1, promoting leukemia-cell migration and proliferation, respectively. By binding a functional epitope in the extracellular domain of ROR1, cirmtuzumab can block ROR1-dependent, non-canonical Wnt5a-signaling.
Methods: We conducted a phase 1 trial of cirmtuzumab in patients (pts) with progressive, relapsed/refractory CLL who were not amenable to approved therapies. The primary aims of the study were to evaluate the safety and tolerability of cirmtuzumab and determine the maximum tolerated dose and/or recommended phase 2 dose. Secondary endpoints included evaluation of antibody pharmacokinetics (PK) and pharmacodynamics (PD). To address these endpoints, pts received only 4 biweekly infusions of antibody at doses ranging from 15 mcg/kg to 16 mg/kg in a standard 3+3 pt-per-cohort schema.
Results: As of a planned analysis, 20 pts have received cirmtuzumab. Pts tolerated cirmtuzumab extremely well without any noted drug-related severe adverse events or dose-limiting toxicities. Anemia (7 pts), thrombocytopenia (4 pts), and neutropenia (3 pts) were the most common AEs, were primarily grade 1, and were likely due to late-stage-disease-related cytopenias.
We developed an ELISA assay to measure serum levels of cirmtuzumab capable of binding to the targeted epitope of ROR1. Peak concentrations were detected within one hour after the completion of each infusion. The serum concentrations of active cirmtuzumab increased with each subsequent infusion. We detected significant levels of cirmtuzumab for at least eight weeks following the last infusion and determined that it has half-life of >24 days (d).
Twenty-four hours following the 1st infusion, the leukemic cells of pts treated at doses >/= 2 mg/kg had inactivation of RhoA and Rac1, which were each observed to be activated in all cases prior to therapy. Loss of GTPase activation also was observed for CLL cells sampled at later time points.
Clinically, we observed that most pts had an initial increase in the ALC without evidence of disease progression, similar to what is observed with targeted therapies that inhibit B-cell-receptor/chemokine signaling. Such a redistributive lymphocytosis (defined as an increase in the ALC of 10% or more) was observed in 5 of 9 pts who received doses </=1 mg/kg, and 9 of 11 pts who received doses >/=2mg/kg. The lymphocytosis typically peaked 24 hours after dosing (median 36% increase, range 10-76%), with a subsequent reduction to at or below baseline levels.
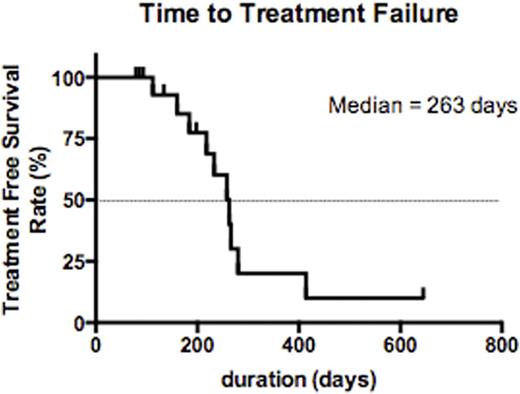
Most pts had stable disease when assessed at the completion of treatment. Of evaluable pts, 12 had stable disease and 2 had progressive disease, both of whom received<1mg/kg per dose of cirmtuzumab. Some patients experienced reductions in lymph node size or in absolute lymphocyte count (ALC) (max 58% reduction from baseline). Strikingly, pts generally had sustained stabilization of disease after receiving just 4 infusions of cirmtuzumab and had a long progression-free survival before requiring other therapy (PFS). The median PFS was 263 d (range 112-414 d). Of note is the steep fall-off in the PFS survival curve after a period equivalent to approximately 7x the half-life of cirmtuzumab, when the plasma antibody concentration would be expected to be negligible.
Conclusions: Cirmtuzumab is well-tolerated and has a long half-life, making it feasible to consider monthly dosing. We found cirmtuzumab has biologic activity in the pts treated, specifically inactivating Rho-GTPases of leukemia cells in vivo. Despite receiving limited treatment, pts had prolonged PFS. Cumulative pre-clinical and phase 1 clinical data suggest that cirmtuzumab mobilizes ROR1-expressing CLL cells, thereby preventing progression in protective niches. These results encourage phase 2 testing of cirmtuzumab administered over a longer duration, either alone and/or in combination with other targeted therapies that have anti-leukemia activity, which might be mitigated by non-canonical, ROR1-dependent Wnt5a-signaling.
Jamieson:Johnson & Johnson: Research Funding; GlaxoSmithKline: Research Funding; CTI Biopharma: Research Funding. Kipps:AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal