Abstract

Introduction: Ibrutinib (Ibr) is a kinase inhibitor (KI) indicated for treating CLL. Clinical trials that led to its approval showed that its unique side effects differ from traditional chemotherapy toxicities. We previously reported (Mato et al, ASH 2015) that intolerance was the most common reason for discontinuation of Ibr in 123 patients treated in a real world setting. Whether reasons for discontinuation reported in clinical trials mirror those encountered in the real world is unknown and has not been studied. Therefore, we conducted a retrospective analysis of 621 CLL patients treated with Ibr either on clinical studies or commercially. We aimed to compare the type and frequency of toxicities reported in either setting, assess discontinuation rates, and evaluate outcomes.
Patients and Methods: This multicenter, retrospective analysis included Ibr-treated CLL patients at 9 US cancer centers or the Connect® CLL Registry. We examined demographics, dosing, discontinuation rates and reasons, toxicities, and outcomes. The primary endpoint was progression-free survival (PFS) (time from KI treatment to progression, death or last f/u) as determined by the Kaplan Meier method. Comparisons of outcomes data were made using the log rank (LR) test. All other comparisons were descriptive.
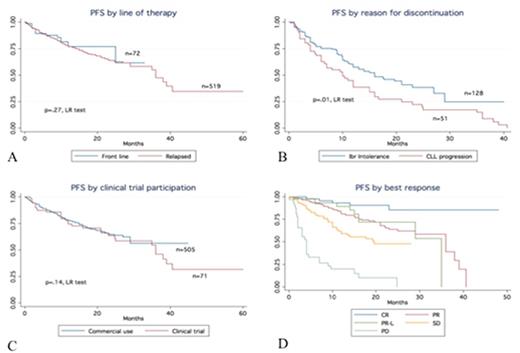
Results: 621 patients treated with Ibr were identified. Table 1 includes available baseline characteristics stratified by line of therapy. A total of 546 (88%) patients were treated with commercial drug. Clinical trial patients were younger (median age 57 vs. 61 years), had a longer time from diagnosis to Ibr (median 85 vs. 72 months) and were more consistently initiated at 420 mg daily (100% vs. 89%). With a median f/u of 14.5 months, the Ibr discontinuation rate was estimated to be 42% (median time to Ibr discontinuation was 7 months). Reasons for discontinuation are listed in table 2. Notably, Ibr toxicity was the most common reason for discontinuation in all settings. Ibr starting dose (420 mg daily vs. < 420 mg daily) did not impact the proportion of patients who discontinued Ibr due to toxicity (51% vs. 50%). In relapsed CLL, the 5 most common Ibr-related toxicities as a reason for discontinuation included: atrial fibrillation (12.3%), infection (10.7%), pneumonitis (9.9%), bleeding (9%), and diarrhea (6.6%). In front line CLL, the 3 most common Ibr-related toxicities as a reason for discontinuation included arthralgia (41.6%), atrial fibrillation (25%), and rash (16.7%). Median times to discontinuation by toxicity were as follows: bleeding (8 months), diarrhea (7.5 months), atrial fibrillation (7 months), infection (6 months), arthralgia (5 months), pneumonitis (4.5 months), and rash (3.5 months). Median PFS and OS for the entire cohort were 35 months and not reached (median f/u 17 months) respectively. Figure 1 describes PFS for Ibr treated patients stratified by line of therapy (A), reason for discontinuation (B), clinical trial participation (C) and depth of response (D). In a multivariable model, complex karyotype was validated as an independent predictor of PFS (HR 1.6, CI 1.1-2.5 p=.04) but not OS (HR 1.6, CI .9-3.1 p=.1).
Conclusions: In the largest reported series on Ibr-treated CLL patients, we show that 40% of patients have discontinued Ibr during this observation period. Intolerance as opposed to CLL progression or transformation was the most common reason for discontinuation. As compared to previous reports from clinical trials, the discontinuation rate appears to be higher suggesting (1) a learning curve in terms of toxicity management, (2) a higher incidence of toxicity in clinical practice, (3) or a lower threshold for discontinuation given alternative choices. Outcomes remain excellent and were not impacted by line of therapy and whether patients were treated on studies or commercially. These data strongly argue to find strategies to minimize Ibr intolerance so that efficacy can be further maximized.
Mato:Abbvie, Acerta Pharma, Gilead Sciences, ProNAi, TG Therapeutics, Theradex: Research Funding; Abbvie, Gilead Sciences, Pharmacyclics, TG Therapeutics: Consultancy. Lamanna:Acerta: Research Funding; TGR Therapeutics: Research Funding; Janssen: Honoraria; Pharmacyclics: Honoraria, Research Funding; Roche-Genentech: Honoraria, Research Funding; Celgene: Honoraria; AbbVie: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Infinity: Research Funding; Acerta: Research Funding; TGR Therapeutics: Research Funding. Ujjani:Gilead: Consultancy; Abbvie: Consultancy; Genentech: Consultancy; Pharmacyclics: Consultancy. Brander:TG Therapeutics: Research Funding; Gilead: Honoraria. Howlett:Sandoz: Honoraria; Teva: Speakers Bureau; Amgen: Honoraria; Pfizer: Honoraria; Eisai: Honoraria. Skarbnik:Gilead Sciences: Speakers Bureau; Seattle Genetics: Speakers Bureau; Genentech: Speakers Bureau; Abbvie: Consultancy; Pharmacyclics: Consultancy. Cheson:Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kiselev:Celgene: Employment, Equity Ownership. Nasta:Millennium Pharmaceuticals: Research Funding. Schuster:Janssen Research & Development: Research Funding; Hoffman-LaRoche: Research Funding; Gilead: Research Funding; Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding. Porter:Novartis: Patents & Royalties, Research Funding; Genentech: Employment. Nabhan:Seattle Genetics: Research Funding; Cardinal Health: Consultancy; Infinity: Consultancy; Abbvie: Consultancy; Genentech: Consultancy, Research Funding; Celgene Corporation: Consultancy, Research Funding; Astellas: Research Funding. Barr:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal