Abstract
Introduction: MDS encompasses a heterogeneous group of clonal neoplastic hematopoietic stem cell diseases characterized by ineffective hematopoiesis and frequent progression to acute myeloid leukemia (AML). While the advent of hypomethylating agent (HMA) therapy has modestly improved survival in higher-risk MDS, outcomes following HMA failure are dismal. The hedgehog (HH) signaling pathway is an important regulator of hematopoietic cell survival and differentiation, with upregulation of HH target genes and signaling proteins a frequent occurrence in MDS and AML. PF-04449913 (PF-04) is an orally bioavailable inhibitor of Smoothened (SMO), which demonstrated antileukemic activity in a prior phase 1 study. We performed a phase 2 study of PF-004449913 in patients with MDS following HMA failure.
Objectives: 1) primary: to estimate overall IWG-2006 response rate (CR + PR + HI + marrow CR) to PF-04449913 in patients with relapsed/refractory MDS and CMML; 2) secondary: to assess safety , overall survival, time to AML transformation, and effect of PF-04 on HH target gene expression.
Methods: This was a single center, open-label phase 2 study. Key eligibility criteria included: 1) patients age ≥ 18 with MDS, CMML, or AML (20-29% blasts) following failure of HMA; 2) ECOG 0-2; 3) Adequate kidney and hepatic function. Treatment consisted of PF-04 at 100 mg/day in 4-week cycles for 4 cycles, with continuation allowed for achievement of stable disease or better. Dose increase to 200 mg/day was permitted after cycle 2 for patients with stable disease. Bone marrow biopsy for response assessment occurred after cycle 2, cycle 4, and every 4th cycle thereafter. For correlative studies, total cellular RNA was isolated from ficolled mononuclear cells in baseline marrow samples, and qPCR performed for expression of GLI1, PTCH1, and SMO).
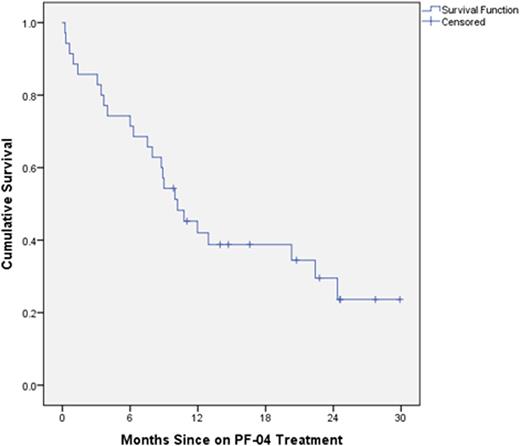
Results: Thirty five patients were enrolled, 24M and 11F. Median age was 75 years (range 55-88). A majority of patients had higher-risk (54%) vs lower-risk (43%) MDS by IPSS at time of enrollment. A total of thirty four patients (97%) had received prior azacitidine for a median of 9 cycles (range 1-88), while 6 patients had received decitabine for a median of 4 cycles (5 of whom had also received azacitidine). The median number of cycles of PF-04 received was 3 (range 1-11). All 35 patients were evaluable for response. Two of 35 patients (6%) achieved response per MDS IWG 2006 criteria, including one patient with HI-N/HI-P and another with marrow CR and HI-N. Nineteen additional patients (54%) had stable disease following cycle 2 (8 weeks). With a median follow-up of 10.1 months, the median survival for all treated patients was 10.2 months (95% CI 6.8-13.6 mo)(Figure 1). Median survival for patients with low/INT-1 risk MDS was longer than for INT-2/high risk patients (22.4 mo vs 7.5 mo, p=0.013). Six patients (17%) died while on-treatment, but none of the deaths were considered related to PF-04. The most common treatment-emergent adverse events included myalgia/muscle cramps (65%), dysgeusia (60%), nausea (31%), anorexia (31%), oral mucositis (25%), and AST elevation (22%). The vast majority of these events were grade 1/2. Except for infection (11%), there were no treatment-emergent grade 3/4 events in > 10% of patients. Only 5 patients (14%) required dose reduction to 50 mg/day due to intolerance. In the pharmacodynamics analysis, responding patients had higher baseline expression of SMO, PTC ,and GLI1 than non-responders, while non-responders had higher post-treatment increases in these transcripts compared with responders.
Conclusions: PF-04 was well tolerated in patients with advanced, HMA refractory MDS, but had limited efficacy in this unselected, heavily pre-treated patient population. The pharmacodynamic analysis suggests predictive potential of HH pathway gene expression for response warranting further investigation.
Komrokji:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy. Sweet:Novartis: Consultancy, Speakers Bureau; Ariad: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau; Incyte Corporation: Research Funding; Karyopharm: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal