Abstract
Introduction
About 75% of myelodysplastic syndromes (MDS) are lower risk categories with a paucity of definitive treatment and only supportive management as an option. MDS, defined by ineffective erythropoiesis produces anemia requiring frequent blood transfusions resulting in systemic iron overload. Refractory Anaemia with ring sideroblasts frequently characterised by mutations in the spliceosome machinery also contributes to cellular iron overload. Excess iron drives activation of the transcription factor NF-κB promoting pro-inflammatory cytokines production supporting tumour growth. Iron chelation is routinely used to treat transfusional iron overload. A number of observational studies have demonstrated that the iron chelator Deferasirox (DFX), improves haemoglobin levels in a subset of MDS patients (Messa, et al. 2008, Banerjee, et al. 2015). Iron metabolism is deregulated in cancer cells resulting in a net iron influx enhancing ROS production. Excess ROS promotes autophagy, a catabolic cellular recycling pathway clearing redundant and damaged organelles to sustain cellular metabolism. Autophagy is initiated by the Atg1-Atg13 protein complex and can be upregulated in cancer facilitating the propagation of the malignant clone. We have previously demonstrated that treatment with the iron chelator DFX, blocked growth of myeloid leukemia cell lines whilst sparing normal stem cells. Herein, we demonstrate that the growth inhibitory effects of DFX are mediated by its ability to inhibit autophagy. We therefore postulate that modulation of intracellular iron levels can be adopted as a viable tool to further elucidate the role of autophagy in this disease.
Aims
To explore the impact of iron modulation on autophagy.
To elucidate the role autophagy in myeloid cell growth and proliferation.
Methods
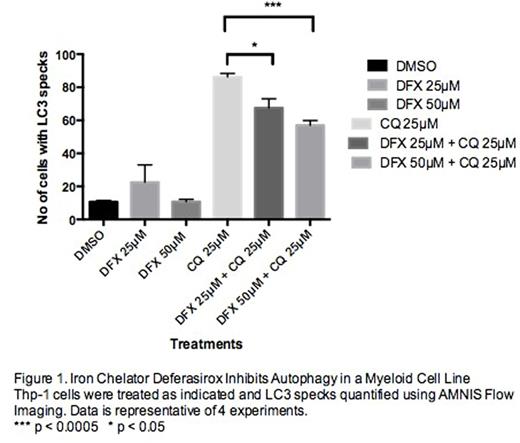
Thp-1 (myeloid cell line) expressing the autophagy reporter LC3-GFP was utilised. Autophagy results in LC3 recruitment to the autphagosomal membrane. These cells were treated with DFX in the presence/absence of chloroquine, a lysosomal enzyme inhibitor to visualise accumulated LC3-II. Cells were also treated with two other iron chelators, deferoxamine (DFO) and Dp44mT. Treated cells were then analysed using AMNIS Flow Imaging. Acute myeloid leukaemia cell lines were treated with DFX in the presence/absence of chloroquine and subjected to Western blotting using anti-LC3-II antibody. AML cell lines were also treated with 3-methyl adenine (3MA), an autophagy inhibitor. Doxycycline inducible CRISPR/Cas9 gene editing was used to delete essential autophagy genes, ATG 5 and 7 in Thp-1 cells and subjected to Western Blotting using ATG5/7 antibodies. Cell proliferation assays were done in the presence and absence of doxycycline. The impact of ATG5/7 deletion on LC3 was analysed using Western Blotting.
Results
CQ treatment of Thp-1-LC3-GFP cells demonstrated an increased number of LC3 specks, which were significantly reduced upon co-treatment with DFX. The total number of cells overall with LC3 specks was also reduced in the combination treatment of CQ and DFX. This was consistent with Western Blotting results that demonstrated lower LC3-II levels in this treatment. Cells treated with the other iron chelators, DFO and Dp44mT demonstrated no decrease in LC3 specks upon co-treatment with CQ and confirmed with Western Blotting demonstrating no change in LC3-II levels. Pharmacological inhibition of autophagy in AML cell lines using 3MA, showed dose dependent decrease in cell growth. ATG5 and ATG7 editing in Thp-1 cells using CRISPR/Cas9 resulted in significant impact on cell growth and proliferation. Loss of ATG5 and ATG7 in these cellswas confirmed using Western Blotting. LC3-II levels were significantly reduced in ATG5/7 deleted cells.
Conclusion
Our results demonstrate an essential role for the autophagy mediators ATG5/7 highlighting the importance of autophagy in promoting proliferation of malignant myeloid cells. The negative impact of DFX on cell growth and proliferation appears to be via its ability to inhibit autophagy. This is a unique property of DFX not observed with other iron chelators. Using DFX as a tool to modulate iron metabolism, these results have reinforced the role of deregulated iron metabolism in propagation of malignant haematopoietic cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal