Abstract
Thrombosis is a major cause of morbidity and mortality in polycythemia vera (PV) and essential thrombocythemia (ET) patients. Known risk factors for thrombosis include: age > 60 years, history of thrombosis, high leukocyte count and JAK2V617F positivity. Several studies have failed to show correlation between increased platelet count and thrombosis, though qualitative abnormalities in multiple blood cell lineages have been shown. The mechanistic basis of thrombosis in PV/ET, however remains unknown. To better understand the pathophysiology of thrombosis in PV and ET, we studied transcript levels of selected thrombotic, inflammatory and hypoxia inducible factor (HIF) pathway genes in granulocytes and platelets of these patients.
Genes selected for the study include: F3 gene encoding tissue factor (TF); P-selectin (SELP); serpin peptidase inhibitor clade E member 1 (SERPINE1, encoding plasminogen activator inhibitor I, PAI1); thrombospondin 1 (THBS1); interleukin 1 receptor associated kinase 1 (IRAK1); interleukin 1 receptor accessory protein (IL1RAP). We also interrogated these HIF regulated genes: vascular endothelial growth factor A (VEGFA) and solute carrier family 2 (SLC2A1, encoding glucose transporter 1). Transcript level of these selected genes in granulocytes and platelets was determined by QT-RT PCR using TaqMan Expression Assays (Applied Biosystems, CA).
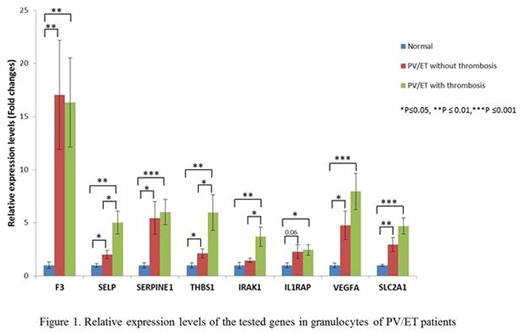
Thirty-three patients with PV (all JAK2V617F positive), and 12 ET patients (7 JAK2V617F , 3 calreticulin mutated and 1 triple negative) were included in the study. There were 25 females and 20 males with a mean age of 60.7 years. Of these, 23 patients had a history of thrombosis (15 females and 8 males). Fifteen patients had abdominal and 8 had other thromboses, including stroke, deep vein thrombosis, pulmonary embolism, cerebral venous sinus thrombosis or arterial clots in microvasculature. In granulocytes, we observed a higher expression of all these genes in PV/ET patients compared to normal controls. Transcript levels of SELP, THBS1 and IRAK1 were elevated in PV and ET patients with thrombosis, compared to those who did not have thrombosis (Figure 1). In platelets, all the tested genes had higher transcript levels in PV/ET patients compared to controls, except IRAK1, which had lower expression. Thrombotic patients had higher transcripts of F3, SERPINE1, IL1RAP, VEGFA and SLC2A1 than those without thrombosis(Figure 2). We are now comparing TF activity with its F3 transcript and the effects of therapy with hydroxyurea and pegylated interferon on the expression of these genes.
TF is a principal initiator of coagulation and we found high levels of F3 transcripts in PV/ET patients by QT-RT PCR. We also found, in a different cohort of PV patients, increased transcripts of F3 in granulocytesby unbiased RNA sequencing. However, monocytes are the principal source of TF (Osterud, Thromb Res. 2010), and indeed, control monocytes we evaluated had higher transcripts of F3 than granulocytes or platelets. Unlike controls, it is possible that granulocytes and platelets may transcribe F3 in PV/ET patients. In ongoing analysis, the expression levels of F3, and TF activity in PV/ET monocytes is being evaluated.
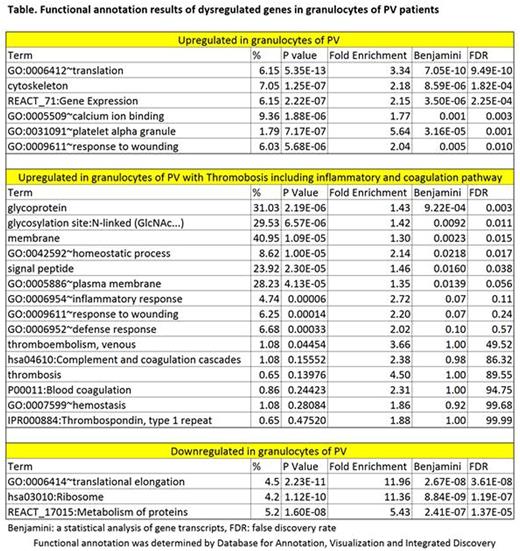
We also analyzed the whole transcriptome in a different cohort of PV patients (9 with and 16 without thrombosis) by RNA Seq. In this cohort, granulocyte analysis revealed that 2,025 genes were overexpressed and 2,137 genes downregulated. Functional annotation results are shown in the Table. In PV patients with thrombosis, 644 genes were upregulated and 573 genes downregulated. About 10 % of these upregulated genes are associated with inflammation and coagulation pathways, including SERPINE1.Alternative splicing variants of SELP, THBS1 and IL1RAP were detected. Alternative splicing variants with aberrant expression were found in 884 genes in PV, and 35 genes in PV patients with thrombosis. Analysis of platelets is ongoing.
In conclusion, transcripts of thrombo-inflammatory and HIF-regulated genes are increased in granulocytes and platelets in PV and ET patients, which may play a role in increased thrombotic risk. The transcripts of these genes differ in granulocytes and platelets, reflecting the fact that their transcription is tissue specific. The differences in gene expression may identify patients with higher risk for thrombosis and possible therapeutic targets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal