Abstract
Backgrounds: Primary myelofibrosis (PMF) is associated with poor prognosis and a marked reduction in life expectancy, with median survival durations ranging from 3.5 to 6 years, according to previous studies with great variation. We conducted a 17-year nationwide survey (1999-2015) to elucidate the clinical outcomes of patients with PMF in Japan, and evaluated the change of survival duration over time.
Patients and Methods: Questionnaires were sent annually to approximately 500 hematology departments of board-certified member institute of the Japanese Society of Hematology to collect data about clinical features and prognoses of patients with PMF. Newly diagnosed patients with PMF were enrolled in this study and were followed up annually to collect prognostic information. Approximately 50 patients were enrolled per year, yielding an eventual total of 780 patients with PMF that were included in a 17-year study period (1999-2015).
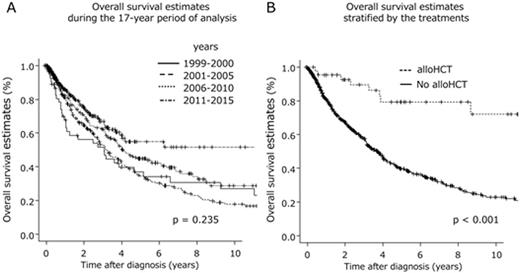
Results: The median age at diagnosis was 66 years. At the time of the analysis, 374 enrolled patients (48%) had died after a median survival duration of 47 months (95% CI, 41-53 months). Of the patients for whom the final cause of death was known, infection (n = 89) and transformation into acute leukemia (n = 91) were the most frequent causes. The 3-year overall survival (OS) rate was 59% (95% CI, 55%-63%). Significant improvements in the 3-year OS rate were not observed during the 17-year period of analysis (p = 0.235)(Figure A). Among the proposed prognostic models for predicting the outcomes of PMF patients, the Dynamic International Prognostic Scoring System of PMF (DIPSS) plus model was the most feasible for our cohort. However, the calculated areas under ROC curves were 0.609, and only modest accuracy was observed in this regard. Forty-three patients (median age of 52 years; range, 24-66 years) received allogeneic hematopoietic stem cell transplantation (alloSCT) at a median of 343 days after diagnosis. The estimated median survival duration after first alloSCT was 134 months (range, 71 to not reached), with a 3-year OS rate of 84% (95% CI, 68%-93%). The median survival duration after diagnosis among patients who received alloSCT was 207 months (range, 105 to not reached), and this is significantly longer than that calculated for patients who did not receive alloSCT (median: 45 months; range, 38-49 months; p < 0.001)(Figure B). Ruxolitinib therapy was given to 47 patients, but did not have an impact on survival duration.
Conclusions: The survival curves of patients diagnosed during different subperiods of our study period exhibited complete overlap, indicating a lack of improvement in survival duration over time. However, only alloSCT among current treatment modalities had an impact on the natural course of PMF. Regarding prognostic prediction, DIPSS plus was the optimal model for survival prediction even among Japanese patients with PMF. However, the ROC analysis indicated that the prognostic covariates in DIPSS plus were not sufficient, and additional information regarding gene mutation statuses (e.g., CALR and ASXL1) might be required to predict the precise outcomes of PMF patients. A long-term registration study is required for further evaluations of prognosis and the impact of treatments on survival.
Shibayama:Novartis Pharma: Honoraria, Research Funding, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; Takeda: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Ono Pharmaceutical: Speakers Bureau. Ozawa:Sumitomo Dainippon Pharma Co. Ltd: Research Funding; Takara Bio Inc: Research Funding; Celgene Japan: Consultancy; JCR Pharmaceutical Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal