Abstract
BACKGROUND: Myeloproliferative neoplasms (MPN), including polycythemia vera (PV), essential thrombocythemia (ET), and primary meylofibrosis (PMF), are frequently associated with splanchnic vein thromboses (SVT). Risk factors for SVT in MPN patients differ from risk factors for all-cause thrombosis. This is likely due to differing disease mechanisms at play, and suggest a separate disease phenotype for MPN/SVT patients. While several studies have characterized the features of MPN patients with SVT, a direct comparison of MPN/SVT versus all MPN patients has been lacking.
METHODS: We performed a retrospective, cross-sectional analysis of patients at Barnes-Jewish Hospital from 2000-2014 with MPN and SVT. Patients were identified using ICD-9 codes in the electronic medical record. 52 available patients with both MPN and SVT were included. Randomly selected 134 patients with MPNs only were used as controls. Clinical and laboratory variables were compared between the two groups. Quantitative JAK2 V617F allele burdens were available in 20 patients. As continuous variables were not normal in distribution, a Mann-Whitney U test was performed. Non-continuous variables were compared with an N-1 Chi-squared test to accommodate rare events. All p-values were corrected for multiple testing.
RESULTS: MPN/SVT patients were significantly younger at time of MPN diagnosis (median age 47 vs 57 years, p=0.003). MPN/SVT patients were more likely to have splenomegaly (83% vs 30%, p=0.003), deep vein thrombosis (37% vs 15%, p=0.003), and concomitant thrombophilia (17% vs 2%, p=0.003). MPN/SVT patients had a higher proportion of females (63% vs 54%), but this finding did not reach significance. However, PV/SVT patients had a significantly higher proportion of females compared to PV alone (67% vs 37%, p=0.02). There were no significant differences in JAK2 mutation status, race, smoking status, presence of stroke or coronary artery disease risk factors.
MPN/SVT patients had significantly lower hemoglobin (13.1 vs 14.6, p=0.024), hematocrit (39.3 vs 43.5, p=0.027), and platelet count (513 vs 698, p=0.003) at time of MPN diagnosis. When analysis was restricted to PV, only hemoglobin (14.6 vs 17.24, p=0.007) and hematocrit (44.3 vs 50.68, p=0.012) were significantly lower in SVT patients. No significant differences in cell counts were detected in ET and PMF patients.
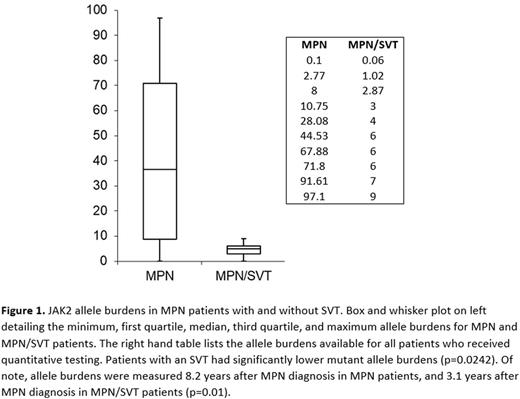
MPN/SVT patients had significantly lower JAK2 mutant allele burdens, with no MPN/SVT patient having an allele burden greater than 10% (p=0.019) (Figure 1). In contrast, mutant allele burdens for MPN patients ranged from 0.1 to 99.7%, with median allele burden being 36.3%.
DISCUSSION: This is the first study to directly compare clinical and laboratory features of MPN patients with and without SVT. Our results confirm that MPN/SVT patients are younger, and within PV are more likely to be female. We also demonstrate that MPN/SVT patients have lower cell counts and lower JAK2 mutant allele burdens, findings not previously shown in the literature. MPN/SVT patients are more likely to have splenomegaly, concurrent thrombophilia, and additional DVT.
These results indicate that MPN/SVT patients exhibit a disease phenotype distinct from MPN patients without SVTs. While the nature of this study is retrospective and causality cannot be definitively established, the findings of younger age, lower laboratory values, and lower JAK2 allele burden are consistent with the hypothesis that MPN/SVT patients present early in disease. It is possible that in MPN/SVT patients, other environmental and host factors (such as concurrent thrombophilia), in combination with early MPN disease, result in the first manifestation of SVT. These findings have important implications, as investigating the natural course of MPN/SVT patients would allow insight into MPN disease pathogenesis.
These findings also suggest that SVTs in MPN patients are not solely mediated by elevated cell counts. In addition, while the presence of the JAK2 V617F mutation likely does affect thrombotic risk, the finding of lower allele burdens in MPN/SVT patients suggests that additional interactions mediate SVT development. These interactions are likely multifactorial and include both environmental and genetic factors. Of particular interest would be the presence of yet unidentified driver mutations present in MPN/SVT patients.
Oh:Incyte: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal