Abstract
Background: The phase 3 COMFORT trials demonstrated that the Janus kinase (JAK)1/JAK2 inhibitor RUX reduces spleen volume, prolongs overall survival (OS), and improves MF−related symptoms and measures of quality of life in patients with intermediate-2 or high-risk MF, compared with either placebo (COMFORT-I) or best available treatment (BAT; COMFORT-II). Many patients with MF are anemic or transfusion-dependent; the impact of these features on clinical outcomes is unknown. We evaluated the relationship between transfusion requirement and clinical outcomes in patients treated with RUX in the COMFORT studies.
Methods: Analyses of data pooled from COMFORT-I and -II were stratified by baseline anemia status (defined as receiving ≥2 units of red blood cells [RBCs] within the 12 weeks before baseline or baseline hemoglobin [Hb] <10 g/dL). Transfusion independence was defined as the absence of RBC transfusions and maintenance of Hb levels ≥8 g/dL for ≥12 weeks; transfusion dependence was defined as a requirement for ≥4 units of RBCs or Hb levels <8 g/dL during an 8-week interval (Gupta V, et al. JCO [ASCO Abstracts]. 2015;33[s15]:abstract TPS7102). Patients achieving transfusion independence during Weeks 13-24 were considered responders for independence by Week 24; those developing transfusion dependence during Weeks 17-24 were considered dependent by Week 24. Effects of transfusion status at Week 24 on MF Symptom Assessment Form total symptom scores (TSS), spleen volume, and body weight were assessed descriptively. The effect on OS was evaluated using the landmark approach (including patients completing ≥24 weeks of study treatment) with stratified log-rank tests for responder vs nonresponder comparisons. Times to first occurrence of transfusion independence and first occurrence of transfusion dependence in the ITT population (censored at last clinical visit), and time to discontinuation among patients in the RUX group who were anemic at baseline (censored at Week 240) were analyzed using the Kaplan-Meier method.
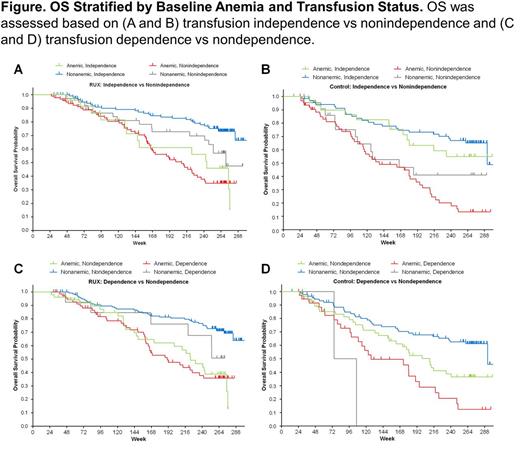
Results: Overall, 301 patients were randomized to RUX (baseline anemia, n=138 [45.8%]) and 227 to the control group (placebo or BAT; baseline anemia, n=113 [49.8%]). In the RUX group, a greater proportion of patients who were nonanemic at baseline (range, 73.4%-73.8%) achieved transfusion independence compared with those who had anemia at baseline (range, 15.5%-22.4%). Week 24 transfusion independence vs nonindependence status did not significantly affect OS in the RUX group (P=0.1322; Figure A), whereas it was significant (P=0.0004; Figure B) in the control group. Similarly, Week 24 transfusion dependence vs nondependence status did not significantly affect OS in the RUX group (P=0.4547; Figure C), whereas it was significant (P=0.0323; Figure D) in the control group. Median OS was significantly longer in the RUX vs control group for patients who were not transfusion independent (baseline anemia, 200 vs 137 weeks; nonanemic, 271 vs 166 weeks; overall P=0.002) or became transfusion dependent (baseline anemia, 210 vs 127 weeks; nonanemic, 292 vs 90 weeks; overall P=0.0323). Changes from baseline in spleen volume, body weight, and TSS at Week 24 were not affected by transfusion or anemia status in the RUX group; however, TSS worsened in the control group among patients who did not achieve transfusion independence vs those who did. Risk of transfusion dependence decreased after Week 24 in the RUX group. The probability of becoming transfusion independent after 1 year of treatment was similar in both treatment groups (approximately 0.75); median time to transfusion independence for the RUX and control groups was 16.6 and 12.0 weeks, respectively. For patients in the RUX group who became transfusion dependent, the mean monthly units of RBCs peaked at Week 20 (2.82 units), decreasing thereafter to 0.52 units at Week 240. Transfusion dependence did not affect RUX discontinuation rates or dosage.
Conclusion: Transfusion requirement had little impact on clinical outcomes or treatment discontinuation within the RUX group but was associated with reduced OS and worsened TSS in the control group. The risk of becoming transfusion dependent, units of RBCs administered, and the monthly proportion of patients requiring transfusions decreased rapidly after 24 weeks of RUX treatment.
Gupta:Novartis: Consultancy, Honoraria, Research Funding; Incyte Corporation: Consultancy, Research Funding. Verstovsek:Bristol-Myers Squibb: Research Funding; Pfizer: Research Funding; CTI BioPharma Corp: Research Funding; Galena BioPharma: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding; Geron: Research Funding; Lilly Oncology: Research Funding; AstraZeneca: Research Funding; Roche: Research Funding; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding. Paquette:Bristol-Myers Squibb: Research Funding, Speakers Bureau; Ariad: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Kiladjian:AOP Orphan: Research Funding; Novartis: Research Funding. Cervantes:AOP Orphan: Membership on an entity's Board of Directors or advisory committees; Baxalta: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sun:Incyte Corporation: Employment, Equity Ownership. Gao:Incyte Corporation: Employment, Equity Ownership. Langmuir:Incyte Corporation: Employment, Equity Ownership. Gopalakrishna:Novartis Pharma AG: Employment, Equity Ownership. Harrison:Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses, Research Funding, Speakers Bureau; Shire: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Baxaltra: Consultancy, Honoraria, Speakers Bureau; Incyte Corporation: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal