Abstract
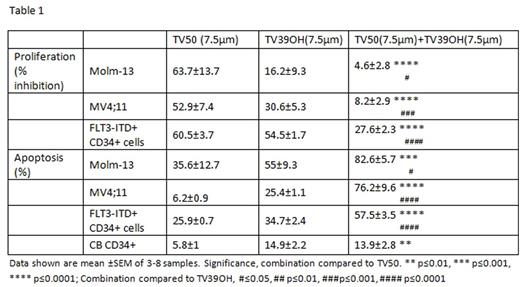
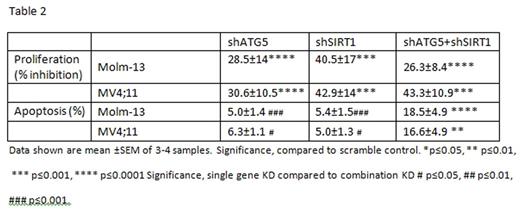
Internal tandem duplication mutations of the Fms-like tyrosine kinase 3 (FLT3-ITD) are amongst the most common mutations in acute myeloid leukemia (AML) patients, and are associated with poor clinical outcome. Tyrosine kinase inhibitors (TKI) targeting FLT3 are only partially effective and fail to yield full and lasting responses in FLT3-ITD+ AML patients. Therefore, additional strategies to enhance eradication of FLT3-ITD+ AML cells are required to improve outcomes. We have previously shown that the NAD-dependent SIRT1 deacetylase is selectively overexpressed in human FLT3-ITD AML leukemia stem cells (LSC), which demonstrate increased sensitivity to the small molecule SIRT1 inhibitor Tenovin-6 (Li at al., Cell Stem Cell 15, 431, 2014). We have now determined that Tenovin-6, in addition to SIRT-1 inhibition and activation of p53 activity, also functions as an effective inhibitor of autophagic flux, mediated by an aliphatic tertiary amine side chain that mediates alkalization of lysosomes. Comparison of Tenovin (TV) analogs with or without SIRT1 or autophagy inhibitory properties revealed that TVs can kill melanoma cells independently of SIRT1 inhibition and that autophagy blockade correlates with ability to completely eliminate cancer cells. Autophagy is known to promote survival and facilitate tumorigenesis and drug resistance by degradation and recycling of intracellular proteins and organelles in lysosomes. In the present work, we investigated the roles of SIRT1 inhibition and autophagy inhibition in targeting AML cells. The TV analogs studied includedTV-39 (inhibits both SIRT1 and autophagy), TV-39OH (inhibits SIRT1 alone), TV-50 (inhibits autophagy alone), and TV-50OH (inhibits neither). We observed that FLT3-ITD + AML cell lines (MV4-11 and MOLM13) demonstrated increased sensitivity to TV analogs with either autophagy inhibitory (TV-50) or SIRT1 inhibitory (TV39OH) effects compared with FLT3-WT cell lines (OCI-AML3 and KG1A). The combination of TV50 and TV39OH resulted in synergistic cytotoxicity in FLT3-ITD+ AML cell lines, as well as in primary AML CD34+cells bearing the FLT3-ITD mutation (Table 1). Western blot analysis indicated that FLT3-ITD+ cells treated with TV39 showed accumulation of LC3B-II, consistent with blockade of a late stage of autophagy, as well as increased acetylated and total p53 expression, consistent with SIRT1 inhibition. Cells treated with TV39OH showed increased acetylated and total p53 without change of LC3B-II levels, whereas cells treated with TV50 alone showed only increase of LC3B-II and not in p53 levels. Knockdown (KD) of p53 using lentivirus-expressed shRNA constructs reversed the inhibitory effects of TVs with both SIRT1 and autophagy inhibitory properties. The combination of the FLT3 TKI AC220 with either TV39OH or TV50 resulted in synergistic cytotoxicity in MV4;11 and Molm-13 cells, indicating that these agents could induce enhance elimination of FLT3-ITD+ cells by TKIs. To confirm the interaction of SIRT1 and autophagy inhibition in targeting FLT3-ITD AML cells, we knocked down ATG5 and SIRT1 expression in MV4-11 and Molm13 cells using lentivirus expressed shRNA. KD of either ATG5 or SIRT1 individually resulted in significant induction of apoptosis and inhibition of proliferation in AML FLT3-ITD cell lines compared with shRNA controls (Table 2). The combined knockdown of SIRT1 and ATG5 induced further increase in apoptosis compared to KD of individual genes. KD of SIRT1 or ATG5 significantly enhanced levels of ROS in FLT3-ITD positive cell lines, measured using the CellROX® Deep Red Reagent (shCtrl vs. shATG5+shSirt1, 1 vs1.74 p=0.02; shCtrl vs shATG5, 1vs 1.83, p=0.009; shCtrl vs. shATG5, 1 vs. 1.85 p=0.008). In an ongoing experiment, we are checking the effect of KD of SIRT1, ATG5 or of both SIRT1 and ATG5 in Molm13 cells in their growth in NSG mice. We conclude that combined inhibition of SIRT1 activity and autophagic flow synergistically targets FLT3-ITD+ AML cell lines and primary CD34+ cells, and enhances their targeting by FLT3 kinase inhibitors. Combined targeting of autophagic flow and SIRT1 activity appears to be a promising strategy to enhance elimination of FLT3-ITD+ AML stem cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal