Abstract
Background
Tyrosine kinase inhibitors (TKIs) are the standard of care for patients (pts) with CML-CP. The current recommendation is to continue TKI therapy indefinitely.1 Results of several clinical trials indicate that pts achieving sustainable deep molecular response (DMR; defined as molecular response ≥ MR4) on imatinib (IM) may achieve long-lasting TFR. Nilotinib (NIL) at 300 mg bid induces higher rates of DMRs compared to IM.2 Also, DMRs can be achieved in pts with NIL (400 mg bid) who are switched after long-term IM.3 However, the optimal duration of consolidation treatment with NIL to increase the chances for successful and continuous TFR (≥ MR4) after stopping treatment is not yet known.
Objective
ENESTPath was designed to assess the proportion of pts (pretreated with IM and subsequently treated with NIL 300 mg bid) who can achieve a sustained DMR and maintain TFR without relapse for 12 months (mo) upon treatment discontinuation after different durations of treatment in consolidation phase.
Methods
ENESTPath is a randomized, phase 3 study enrolling pts with CML-CP who achieved a complete cytogenetic response (CCyR), but not MR4, after at least 24 mo of treatment with IM. After enrollment, pts were assigned to receive NIL at 300 mg bid for either 24 mo or 36 mo (arm 1 and arm 2, respectively). Pts with stable MR4 or better for at least 12 mo will enter the TFR phase. A stable MR4 was defined by 4 of the 5 preceding quarterly real-time quantitative RT-PCR (RQ-PCR) assessments ≥ MR4 and ≥ MR4 inthe last assessment performed by IS certified EUTOS laboratories.
Results
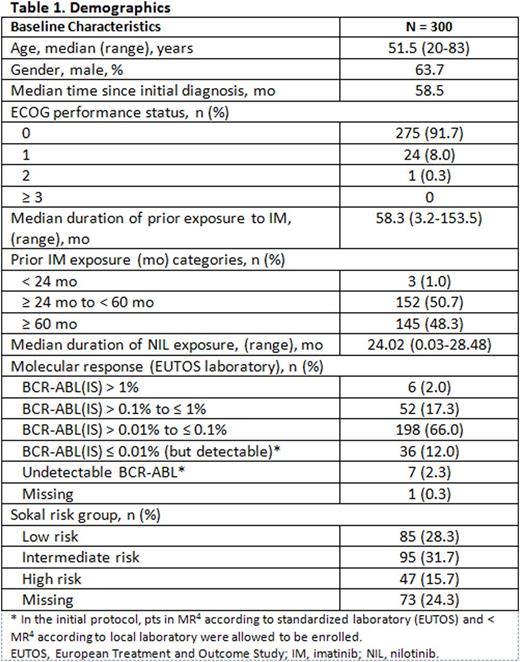
A total of 619 pts were enrolled in the study between May 2013 and June 2015. The present analysis reports the results of the first 300 pts (mean age, 50.8 years; 63.7% of males) enrolled and treated with NIL for 24 mo in induction and consolidation phase or who had discontinued earlier. Details of the baseline characteristics are given in Table 1.
At data cutoff, 108 pts were in stable MR4; 101 (33.7%) were randomized and 7 (2.3%) were scheduled for randomization at that time point. 192 pts (64%) were not eligible for randomization, primarily due to lack of stable MR4 in 126 pts (42%), adverse events (AEs) or abnormal laboratory values in 44 pts (14.7%), and for other reasons in 22 pts (7.3%).
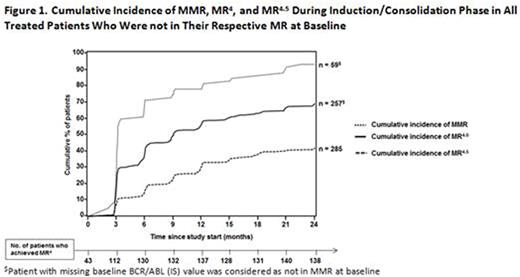
The rates of MR4 at baseline*, 6 mo, 12 mo, 18 mo, and 24 mo were 14.3%, 43.3%, 45.7%, 43.7%, and 46%, respectively. By 24 mo of treatment with NIL, cumulative incidences of major molecular response (MMR), MR4, and MR4.5 of all treated pts not in respective MR at baseline were 93.2%, 69.3% and 42.1%, respectively (Figure 1). Further analysis showed that pts with MMR at baseline had a higher probability of achieving an MR4 than those lacking MMR at baseline, with a cumulative incidence of MR4 by 24 mo of 75.8% and 44.2%, respectively.
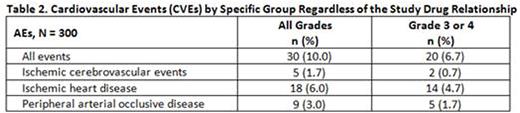
No new safety signals were observed during the 24 mo consolidation with NIL. The majority of the AEs were low grade. Most common AEs irrespective of the relationship to the study drug were pruritus (19%), hypercholesterolemia (14.0%), rash (10.7%), asthenia (10%), and arthralgia (10%). The most common newly occurring or worsening all-grade biochemistry laboratory abnormalities included increase in total cholesterol (68.7%), increased ALT (54%), hyperglycemia (32.7%), and hyperbilirubinemia (37%); majority of them were grade 1 and 2. Newly occurring or worsening all-grade cytopenias include anemia (12.7%), thrombocytopenia (2.3%), leukopenia (2%), and neutropenia (1%). Grade 3 or 4 cardiovascular events (CVEs) were experienced by 6.7% of pts including ischemic heart disease (4.7%), peripheral artery occlusive disease (1.7%), and ischemic cerebrovascular events (0.7%) (Table 2).
Conclusions
This analysis of the first 300 pts after 24 mo of NIL treatment showed a cumulative incidence of MR4 in ~ 70% of pts who were not in MR4 at baseline with an advantage in favor of MMR at baseline. 108 pts (36%) were with stable MR4 at data cutoff and 192 pts (64%) discontinued the study due to very stringent protocol definitions of eligibility for randomization and AEs. Grade 3 or 4 AEs were consistent with the previous reports.4
References
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia V.1.2016 ©2016 National Comprehensive Cancer Network, Inc.
2. Hochhaus A, et al. Leukemia. 2016;30:1044-1054.
3. Hughes TP, et al. Blood. 2014;124:729-736.
4. Rea D, et al. Blood. 2015;125:[abstract 4040].
Rea:Ariad: Honoraria; Pfizer: Honoraria; BMS: Honoraria; Novartis: Honoraria. Rosti:Roche: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Research Funding, Speakers Bureau; Incyte: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Cross:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Niederwieser:Novartis: Research Funding, Speakers Bureau; Amgen: Research Funding, Speakers Bureau. Pregno:Novartis: Honoraria; BMS: Honoraria; ARIAD: Honoraria. Orlandi:Ariad: Honoraria; BMS: Honoraria; Novartis: Honoraria. Almeida:Celgene: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; BMS: Speakers Bureau; Shire: Speakers Bureau; Alexion: Speakers Bureau. Illes:University of Debrecen faculty of medicine department of hematology: Employment. Sagues:ICO-Girona/hospital Universtiari de Girona Dr. Josep Trueta: Employment. Haenig:Novartis: Employment. Supekar:Novartis: Employment. Shah:Cognizant: Employment; Novartis: Other: Vendor. Saglio:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Steegmann:Aria: Honoraria, Other: Research funding for Spanish CML Group; BMS: Honoraria, Other: Research funding for Spanish CML Group; Novartis: Honoraria, Other: Research funding for Spanish CML Group; Pfizer: Honoraria, Other: Research funding for Spanish CML Group. Baccarani:Pfizer: Consultancy, Honoraria, Speakers Bureau; Ariad: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal