Abstract
Background: Timely molecular monitoring is the cornerstone of chronic myeloid leukemia (CML) treatment guidelines. These guidelines are based on the design of clinical trials, but none have been validated prospectively. We hypothesized that timely molecular monitoring in routine patient care increases the likelihood of achieving major molecular response (MMR) in CML.
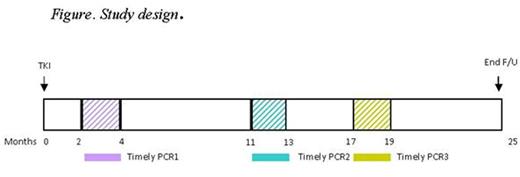
Methods: We conducted a prospective cohort study using the Québec CML Registry, which comprises 713 patients from 16 hospitals. Patients with newly-diagnosed CML (2009-2014) and measurable disease by quantitative PCR were followed from tyrosine kinase inhibitor (TKI) initiation. Timely PCR (tPCR) was defined as a PCR performed at 2-4, 11-13, and 17-19 months. (Figure). Study outcome was the achievement of MMR at 25 months, defined as international scale ratio (IS) <0.1% or a 3-log reduction in BCR-ABL1 copy number. Achievement of MMR was determined using any PCR during follow-up. Generalized estimating equations (GEE) using an exchangeable correlation structure, to account for patient clustering by center, were used to estimate odds ratios (ORs) with 95% confidence intervals (CIs) of achieving MMR comparing adherence and nonadherence to tPCR. The models were adjusted for age, sex, first-line TKI, year of study entry, and Charlson comorbidity index.
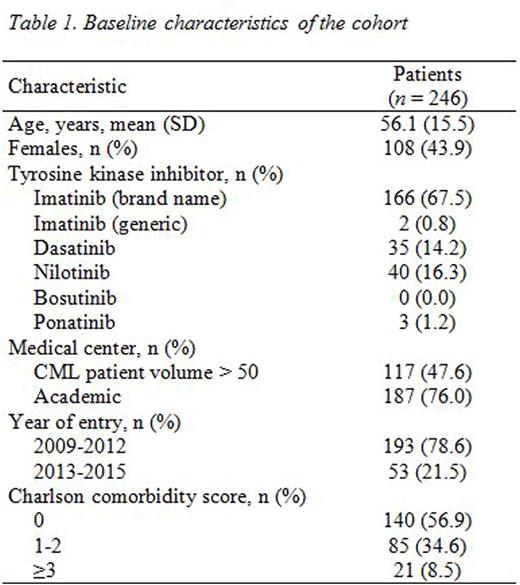
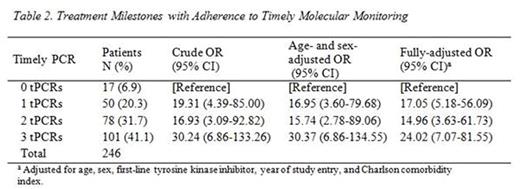
Results: A total of 246 patients with 25 months of follow-up were included in the analysis (Table 1). Patients were excluded due to diagnoses before 2009 (350), insufficient follow-up (76), and other (41). The mean (standard deviation) age was 56.1 (15.5), 43.9% were female; 67.5% were started on imatinib, and 47.6% were treated in higher-volume (>50 CML patients) centers. Timely PCRs were performed in 76.3%, 69.5%, and 61.0% of patients at 2-4, 11-13, and 17-19 months, respectively. When compared with not performing tPCRs, performing one and two tPCRs were associated with achieving an MMR by 25 months (OR: 17.05, 95% CI: 5.18-56.09 and OR: 14.96, 95% CI 3.63-61.73, respectively, Table 2). The highest OR of achieving MMR was observed among those who underwent three tPCRs (OR: 24.02, 95% CI: 7.07-81.55).
Conclusions: To our knowledge, this is the first study to assess clinical outcomes associated with timely molecular monitoring in early CML. While performing one and two tPCRs was associated with achieving MMR at 25 months, the point estimate for performing three tPCRs was the highest. These findings indicate that timely monitoring may allow for faster switching of TKI, which ultimately permits patients with early failure to "catch up." Alternatively, more regular testing may increase patient adherence to therapy. If replicated, these findings support routine and punctual monitoring of patients on TKI therapy.
Assouline:Pfizer: Speakers Bureau; BMS: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal