Abstract
Ribavirin, an antiviral drug used to treat infections including respiratory syncytial virus (RSV), inhibits the eukaryotic translation initiation factor 4E (eIF4E). EIF4E exports key mRNA transcripts from the nucleus and is a critical factor for translation of mRNAs into protein. Prospective trials of patients treated with ribavirin indicate that the drug has clinical activity and expected molecular effects of eIF4E inhibition in AML including relocalization of eIF4E to cytoplasm and decrease in eIF4E levels (Assouline Blood 2009 and Assouline Haematologica 2015). We demonstrated in pre-clinical models including a PDX triple hit lymphoma that eIF4E is also implicated in the pathogenesis of lymphomas (Culjkovic-Kraljacic Blood 2016).
To elucidate the mechanism of action of ribavirin in DLBCL, we conducted eIF4E-immunoprecipitation followed by RNA-sequencing (RIP-seq) in OCI-Ly1 cells to identify RNAs that bind to eIF4E. We integrated this data with the RNA-sequencing of ribavirin-treated OCI-Ly1 cells (vs. vehicle) to further characterize RNAs that are more likely to be decreased by ribavirin. We performed pathway analysis with this data and found several lymphomagenic eIF4E transcripts to be significantly reduced by ribavirin treatment including the BCR, epigenomic regulators, interleukin signaling (IL-6, IL10), DNA damage response elements, and components of the splicing machinery. This suggests that ribavirin interferes with critical pathways in proliferating DLBCL cells and may be active in lymphoma patients.
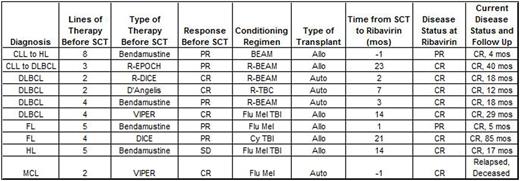
After observing a patient with an aggressive, refractory transformed lymphoma (CLL to HL) demonstrate an objective response on imaging following administration of ribavirin for RSV in absence of concurrent chemotherapy, we retrospectively analyzed (with IRB approval) outcomes of lymphoma patients undergoing autologous or allogeneic SCT who received ribavirin for antiviral indications. We searched our institutional electronic record system and SCT database for lymphoma patients meeting prospectively defined criteria as receiving ribavirin within 6 months prior to or any time after SCT. Ten patients were identified including 5 DLBCL (1 transformed from CLL and another from FL), 2 HL (1 transformed from CLL), 2 FL, and 1 MCL. All were male and median age at lymphoma diagnosis was 54 years (range 35-64). Median number of treatments received prior to SCT was 4 (2-8). Four were deemed to have inadequate response (3 with PD and 1 with insufficient PR) after salvage therapy and were treated with bendamustine prior to proceeding to SCT (3 with an investigational high dose regimen). Responses to therapy immediately prior to SCT included 4 CR, 5 PR, and 1 SD. Six underwent allo SCT and 4 auto SCT.
All patients received ribavirin for RSV (4 inhalational, 6 oral) with a median length of treatment of 10 days (5-15). Median interval between SCT and ribavirin was 5 months (-1 to 23). Nine of 10 patients are currently alive with no evidence of lymphoma with a median OS of 17.8 months (4.6-85.5) and median PFS of 11.1 months (2.4-63.8).
These retrospective data from patients with refractory lymphomas treated with ribavirin as antiviral therapy (just prior to or soon following SCT) demonstrate lymphoma-related outcomes superior to those expected based on disease risk profiles (9 of 10 with ongoing CRs). We recognize the limitations of this analysis, as well as potential selection biases and other possible explanations for these findings. However, our observations, in conjunction with preclinical data on eIF4E inhibition, raise the intriguing possibility that ribavirin may have clinically meaningful anti-lymphoma activity. Further assessment of larger numbers of patients, and rationally designed prospective clinical studies of ribavirin are justified and planned.
Martin:Acerta: Consultancy; Novartis: Consultancy; Gilead: Consultancy, Other: travel, accommodations, expenses; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal