Abstract
Background: There are a number of ongoing clinical trials assessing next-generation immunochemotherapy (IC) regimens (i.e. RX-CHOP) in DLBCL. In addition to standard clinical trial workup, some trials require central pathology review and/or molecular phenotyping before treatment assignment and/or initiation of therapy. There is concern that these studies may be biasing patient selection due to missing patients with aggressive disease who require immediate therapy and cannot delay their treatment to enroll on a study. Here we examine clinical characteristics and outcome stratified by the time from diagnosis to initiation of therapy from a large observational cohort of patients with DLBCL from the R-CHOP era.
Methods: Patients were prospectively enrolled in the University of Iowa / Mayo Clinic SPORE Molecular Epidemiology Resource (MER) within 9 months of diagnosis and followed for relapse, retreatment, and death. Clinical management at diagnosis and subsequent therapies were per treating physician. This analysis includes patients with stage II-IV DLBCL or primary mediastinal B-cell lymphoma (PMBCL) who underwent front-line anthracycline based IC; patients with primary CNS lymphoma, PTLD, or a component of low-grade lymphoma were excluded. Time from diagnosis to treatment was defined as the time from date of first lymphoma-containing biopsy to the initiation of IC therapy; delayed therapy was defined as initiating therapy more than 14 days after diagnosis. Event-free survival was defined as time from diagnosis until progression, retreatment, or death due to any cause. EFS24 was defined as progression free status 24 months from diagnosis.
Results: 720 patients with stage II-IV newly diagnosed DLBCL or PMBCL and treated with IC were enrolled in the MER from 2002-2012. Median age at diagnosis was 62 years (range 18-92) and 399 patients (55%) were male. 541 patients (75%) had stage III/IV disease and IPI at diagnosis was 0-1 in 166 patients (23%), 2 in 221 patients (31%), 3 in 222 patients (31%) and 4-5 in 111 patients (15%). 233 of 395 patients (59%) were GCB per Hans. At a median follow-up of 73 months, (range 0-163), 349 (49%) patients had an event and 267 patients died (37%); 37% of patients failed to achieve EFS24.
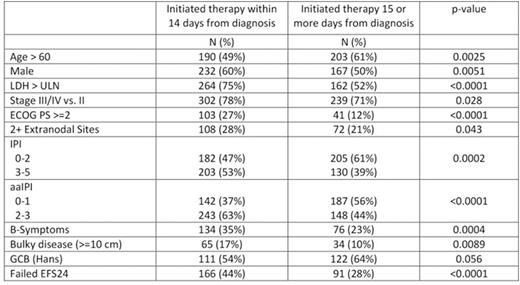
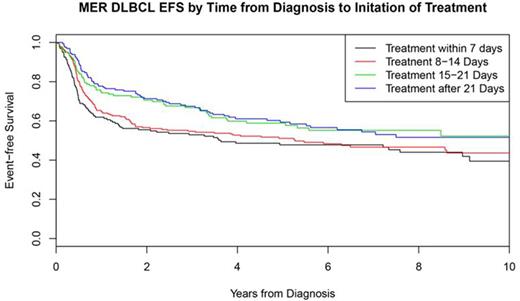
Median time from initial lymphoma diagnosis to initiation of IC was 14 days (range 0-79, IQR=8-23). Patients with delay in therapy (>14 days from diagnosis) were more frequently female (50%, p=0.0051) and older than patients who initiated therapy within 14 days from diagnosis (median age at diagnosis of 63 years vs. 60 years, p=0.0008). Patients with delay in treatment initiation had universally less aggressive disease characteristics, including earlier stage, non-elevated LDH, 0-1 extranodal sites, absence of B-symptoms, lower ECOG PS, and lower IPI (see table). In addition, patients with delayed therapy were enriched for GCB subtype per Hans algorithm (p=0.056). Patients with initiation of therapy within 14 day of diagnosis had significantly worse outcome (EFS24 failure=44%) compared to patients with delayed time to initiation of therapy (EFS24 failure=28%, p<0.0001, figure), which remained significant after adjusting for either IPI or aaIPI (both p<0.005). The association between delayed time to initiation of therapy and EFS24 was observed in both GCB (EFS24 failure = 42% vs. 27%, p=0.019) and non-GCB (EFS24 failure = 46% vs. 27%, p=0.014) subsets by Hans. The lower event rate in delayed therapy patients results in an approximately 10% loss of power if the study was powered based on all patients regardless of timing from diagnosis to therapy.
Conclusions: Patients with delayed therapy from diagnosis have less aggressive clinical characteristics compared to patients who initiate treatment within 14 days from diagnosis. Studies with a lengthy trial work-up including central pathology review period may be selecting patients with less aggressive disease based on the patient's ability to delay treatment to complete study enrollment requirements. Furthermore, selection of patients with less aggressive disease may result in underpowered studies due to a lower event rate (fewer events) than expected. This retrospective analysis would suggest that trials in DLBCL should consider streamlined enrollment and therapy initiation to avoid potential selection bias and loss of power. Further assessment of implications on trial design and outcomes by cell of origin in this setting is ongoing.
Maurer:Kite Pharma: Research Funding; Celgene: Research Funding. Ansell:BMS, Seattle Genetics, Merck, Celldex and Affimed: Research Funding. Nowakowski:Morphosys: Research Funding; Bayer: Consultancy, Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal