Abstract
Introduction: Venetoclax (VEN) is a selective, potent oral BCL-2 inhibitor with clinical activity in patients (pts) with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (NHL). In NHL xenograft models, VEN has enhanced efficacy of CHOP combined with either rituximab (R) or obinutuzumab (G).
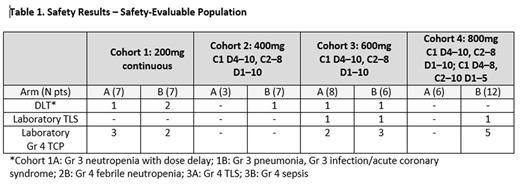
Methods: Dose escalation of VEN + R-CHOP (Arm A) or VEN + G-CHOP (Arm B) was evaluated in B-cell NHL pts with ≤1 prior therapies. Cohort 1 planned for VEN (200mg) on Cycle (C) 1 Day (D) 4, followed by daily administration for eight 21-day cycles. Cohorts 2-4 received VEN (400, 600 and 800mg) on C1 D4-10 and C2-8 D1-10; after the first 6 pts Arm B Cohort 4 dosing regimen was shortened to C1 D4-8 and C2-8 D1-5. CHOP was given for six 21-day cycles; R on D1 of C1-8 (Arm A); G on D1, 8, 15 of C1 and D1 of C2-8 (Arm B). Prophylaxis for tumor lysis syndrome (TLS) at the start of VEN dosing (including hospitalization for mass ≥10cm), mid-cycle complete blood counts, and G-CSF prophylaxis at the start of CHOP cycles were mandatory. Dose-limiting toxicities (DLTs) were assessed during C1-2, the defined DLT period. Objectives included safety, PK, determination of maximum tolerated dose (MTD), recommended phase 2 dose (RP2D) of VEN + R/G-CHOP, and response rate. Response was assessed by PET/CT Lugano criteria, with bone marrow (BM) clearance required for CR in case of baseline positivity. BCL-2 and Myc proteins were assessed by Immunohistochemistry and considered positive if ≥50% of tumor cells showed moderate to strong staining and ≥40% showed nuclear staining, respectively.
Results: As of June 10, 2016, 56 pts were enrolled in Phase I; 91% were previously untreated. Histologies included: FL 24 (43%); DLBCL 17 (30%); MZL 5 (9%); composite lymphoma 5 (9%); and other 5 (9%). Three pts in Cohort 1 had DLT (Table 1), thus the VEN regimen was changed to C1 D4-10 and C2-8 D1-10 for Cohorts 2-4. No protocol-defined MTD was identified up to the maximum assessed dose (800mg) for either arm using intermittent dosing regimen. The most common AEs (all grades) in both arms were neutropenia (46%), nausea (45%), fatigue (38%), diarrhea (36%), and constipation (36%). The most common Gr 3/4 AEs were neutropenia (46%), febrile neutropenia (29%), and thrombocytopenia (TCP) (21%). Fifty out of 56 pts (93%) received G-CSF prophylaxis for neutropenia in C1. Three pts had laboratory TLS after the first VEN dose without any clinical sequelae, which resolved by no intervention (1) or medical intervention including holding dose (2). No cases of clinical TLS were observed. Excluding Cohort 1, Gr 4 laboratory TCP was more frequent in Arm B (8/25 pts; 32%) than Arm A (2/17 pts; 12%); in Cohorts 2-4, TCP occurred mid-cycle, with no associated clinically significant bleeding. As of the data cutoff, 46 pts completed study treatment, of which 33 completed VEN therapy (72%): 20 (83.3%) Arm A, 13 (59%) Arm B. Excluding Cohort 1 (6 pts discontinued VEN), all pts that discontinued VEN due to AEs were on Arm B. Reasons for early VEN discontinuation after Cohort 1 were TCP, pneumonia and sepsis; thus Arm B dose finding is ongoing. Thirty-eight of 46 pts completed CHOP therapy (82.6%): 21 (87.5%) Arm A, 17 (77.3%) Arm B; 7 pts discontinued due to AE and 1 progressive disease (PD) on C1 D5. Of the 42 pts in the intent-to-treat (ITT) analysis that were evaluable for efficacy response (≥1 cycles with available end of treatment (EOT) response assessment or discontinued prior to assessment), 18/21 (85.7%) in Arm A and 17/21 (81%) in Arm B had a response (Table 2). ITT analysis included 5 pts (1 pt Arm A, 4 pts Arm B) that discontinued study treatment early for toxicity (3 in Cohort 1). One pt (Arm A) discontinued study treatment on C1 D5 with PD and later died of PD following salvage chemotherapy. Of the Double Expressor (DE) DLBCL pts, 7/8 (87.5%) responded, 1/8 (12.5%) had a PD (Table 3). Median follow-up for pts with CR/PR at EOT response was 11 months. One of the pts that responded at EOT had progression during follow-up.
Conclusions: VEN when administered for 10 days of the R-CHOP 21-day cycle had an acceptable safety profile. RP2D for VEN + R-CHOP is 800mg C1 D4-10 and C2-8 D1-10, given the acceptable incidence of DLTs. Phase 2 (only frontline DLBCL pts) is ongoing. VEN + G-CHOP dose finding is ongoing due to mid-cycle TCP. Response rates for VEN + R/G-CHOP are promising. High response rate was observed in the poor prognosis population of DE DLBCL. Updated EOT response, follow-up, and safety data will be presented at the meeting.
Zelenetz:Gilead Sciences: Research Funding. Salles:Amgen: Consultancy, Honoraria; Gilead: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Mundipharma: Honoraria. Casulo:Infinity: Consultancy, Honoraria; Celgene: Research Funding. Sehn:roche/genentech: Consultancy, Honoraria; amgen: Consultancy, Honoraria; seattle genetics: Consultancy, Honoraria; abbvie: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria; celgene: Consultancy, Honoraria; lundbeck: Consultancy, Honoraria; janssen: Consultancy, Honoraria. Cartron:Roche: Consultancy, Honoraria; Celgene: Honoraria; Gilead: Honoraria; Jansen: Honoraria. Chamuleau:Roche: Consultancy; Gilead: Consultancy; Celgene: Consultancy. Goy:infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Johnson & Johnson: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Writing support, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Tam:Gilead: Honoraria; Roche: Honoraria; Abbvie: Honoraria; Janssen: Honoraria. Lugtenburg:Celgene: Consultancy; Mundipharma: Consultancy; Servier: Consultancy; Roche: Consultancy; Takeda: Consultancy. Elstrom:Genentech: Employment. Farazi:Genentech: Employment. Liu:Roche: Employment. Szafer-Glusman:Genentech, Inc.: Employment. Mobasher:Genentech, Inc.: Employment. Morschhauser:Celgene: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Janssen: Honoraria; Roche: Consultancy, Honoraria; Servier: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal