Abstract
Purpose.
To evaluate the safety, tolerability and efficacy of the combination of the mTOR inhibitor Temsirolimus and a standard salvage regimen (R-DHAP) in patients with relapsed or refractory diffuse large cell B-Cell lymphoma (DLBCL).
Methods.
This is a prospective, multicenter, phase II, open-label study. Patients with relapsed or refractory DLBCL with a maximum of two prior treatment lines were eligible. The STORM regimen consisted of Rituximab 375 mg/m² (day 2) and DHAP (Dexamethasone 40mg day 3-6, Cisplatine 100 mg/m² day 3, Cytarabine 2x2 g/m² day 4) with Temsirolimus added on day 1 and 8 of a 21 d cycle, with 2-4 cycles planned. In part I, dose levels of 25, 50, 75 and 100 mg for Temsirolimus were predefined. Based on the observed toxicity profile, the independent data safety committee recommended a Temsirolimus dose of 25 mg given on day 1 and 8 for the part II extension cohort of the trial.
Results.
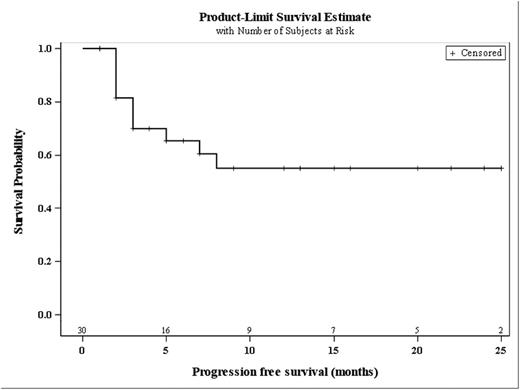
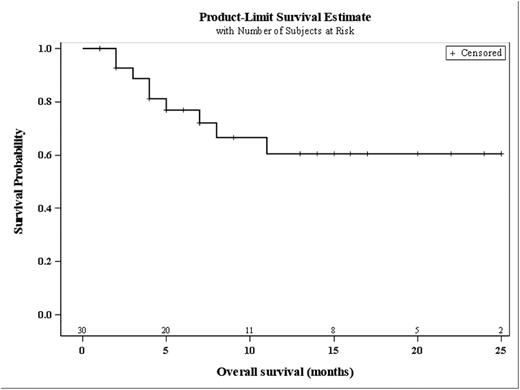
We here report on 46 patients (pts), 15 from part I and 31 from part II. Seven pts were not evaluable for response. Of the evaluable 39 patients, median age was 63 and median number of prior regimen was 1. Temsirolimus dose was 50 mg on day 1 and 8 in 7 pts from the part I of the trial and 25 mg in the remaining 39 pts. The overall response rate was 82% (32/39pts) with 22 partial and 10 complete responses. After a median follow up of 10 months for the total study population, median PFS and OS have not been reached (Figure 1A and 1B). Early safety analysis includes preliminary data of 22 pts. The most frequent non-hematologic side effects were nausea (14 pts, 64%), epistaxis (11 pts, 50%), fatigue (12 pts, 55%), fever (11 pts, 50%) and diarrhea (11 pts, 50%). Frequent grade 3/4 events (n>2) included leukopenia (21 pts, 95%), thrombocytopenia (20 pts, 91%), lymphopenia (11pts, 50%), anemia (8 pts, 36%), neutropenia (10 pts, 45%), renal failure (3 pts, 20%) and infections (7 pts, 32%, bladder infection, esophagus infection, central venous access infection, soft tissue infection, mucositis). Two therapy-related deaths occurred (one patient died from sepsis during neutropenia, another from cerebral bleeding, both events occurring after cycle 3).
Conclusion.
Temsirolimus can be safely added to DHAP and Rituximab with promising activity.
Witzens-Harig:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Viardot:Pfizer: Honoraria; Takeda: Other: travel support; Roche: Honoraria; BMS: Consultancy; Janssen: Consultancy; Amgen: Consultancy. Keller:Roche: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Spectrum Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees. Buske:Celltrion, Inc.: Consultancy, Honoraria. Meissner:Amgen: Other: Travel Support; Takeda: Other: Travel Support; Teva: Other: Travel Support; Celgene: Other: Travel Support. LaRosee:Pfizer: Honoraria. Marks:Pfizer: Honoraria. Hess:Celgene: Honoraria; Roche, CTI, Pfizer, Celgene: Research Funding; Janssen: Honoraria; Roche: Honoraria; Pfizer: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal