Abstract
Background: Patients (pts) with DLBCL who receive first-line anthracycline based immunochemotherapy and remain in remission at 2 years have excellent outcomes with overall survival (OS) in one study that was comparable to the age and sex matched general population in cohorts from the US and France (Maurer et al, JCO 2014). To evaluate whether these findings are robust and generalizable, we assessed OS stratified by progression-free survival at 24 months (PFS24) using individual patient data from pts with DLBCL enrolled in 14 multi-center, international randomized clinical trials as part of the SEAL consortium.
Methods: Central data alignment was performed by the SEAL statistics and data center. All enrolled patients treated with rituximab-containing anthracycline-based immunochemotherapy as part of initial induction therapy were included in this analysis using an intent-to-treat approach. PFS was defined as time from initiation of first-line therapy to progression or death. PFS24 was defined as being alive and progression-free 24 months (731 days) after initiation of therapy. Overall survival from PFS24 was defined as time from achieving PFS24 until death due to any cause. OS was compared to each patient's age-, sex-, and country- matched general population using expected survival and standardized mortality ratios (SMR). Country-level population rate tables were obtained from www.mortality.org where available. A per-study average rate table was used when country information was unavailable. OS beyond 9 years from treatment initiation was only available the time of analysis from two trials (13% of cohort) and thus follow-up from PFS24 was restricted to the first 7 years after PFS24 for this analysis.
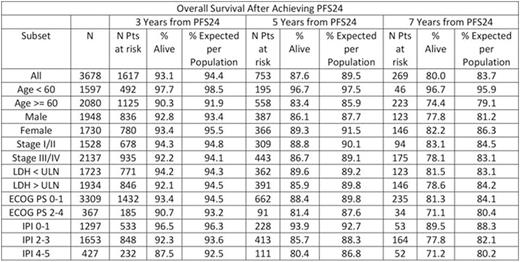
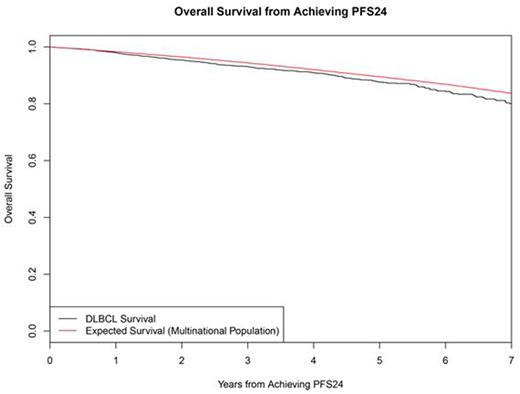
Results: Fourteen studies with 7975 patients were included in the SEAL database. 5853 (73%) received rituximab as part of induction therapy and used in this analysis. Median age was 62 years (range 18-92) with 56% >60 years, 54% were male, 57% had elevated LDH, 63% had stage III/IV disease, 13% had ECOG PS 2-4, and IPI was 0-1 in 36%, 2 in 25%, 3 in 23%, and 4-5 in 16%. At a median follow-up of 4.4 years, 1337 (23%) had disease progression, 1489 patients (25%) had died, and 5101 patients were evaluable for PFS24. 1423 (28%) of evaluable patients failed to achieve PFS24. These patients had poor outcomes after progression with median OS of 7.2 months (95% CI 6.8-8.1), 5-year OS of 19%, and SMR of 32.1 (95% CI: 30.0-34.4). Outcome was excellent (median unreached) in the 3678 patients who were progression-free at 24 months, with subsequent OS after achieving PFS24 approaching the age and sex matched general population (Figure). The observed vs. expected OS at 3, 5, and 7 years after achieving PFS24 was 93.1% vs. 94.4%, 87.6% vs. 89.5%, and 80.0% vs. 83.7%, respectively, the SMR was 1.22 (95% CI: 1.09-1.37). In subset analyses examining pts achieving PFS24, adverse prognostic characteristics at baseline (elevated LDH, advanced stage, ECOG PS 2-4, high IPI) were associated with greater decrement in OS from the general population based expected survival (Table).
Conclusions: PFS24 from the start of initial therapy stratifies DLBCL pts into populations with distinct outcomes. DLBCL pts treated with rituximab containing anthracycline-based immunotherapy on large randomized clinical trials who are alive without progression at 24 months from start of initial therapy have excellent outcomes with survival that is marginally lower but clinically indistinguishable from the age-, sex-, and country-matched background population for at least 7 years after achieving PFS24. Further follow-up time will be needed to assess any long-term impact of late relapse and survivorship issues in this population.
Shi:Mayo Clinic: Employment. Cunningham:Amgen: Research Funding; Astra-Zeneca: Research Funding; Bayer: Research Funding; Celgene: Research Funding; Merrimack: Research Funding; Medimmune: Research Funding. Seymour:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Jaeger:Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Haioun:Sandoz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ghesquieres:Mundipharma: Consultancy; Roche France: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Merli:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Teva Pharmaceuticals Industries: Membership on an entity's Board of Directors or advisory committees. Herbrecht:Servier: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Cell Therapeutics, Inc: Membership on an entity's Board of Directors or advisory committees. Flament:Celgene: Employment, Equity Ownership. Fu:Celgene: Employment, Equity Ownership. Flowers:Gilead: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Acerta: Research Funding; ECOG: Research Funding; Mayo Clinic: Research Funding; Pharmacyclics, LLC, an AbbVie Company: Research Funding; Millenium/Takeda: Research Funding; Infinity: Research Funding; TG Therapeutics: Research Funding; Genentech: Consultancy, Research Funding; NIH: Research Funding; AbbVie: Research Funding. Ou:Mayo Clinic: Employment. Sargent:Celgene: Research Funding. Coiffier:MorphoSys: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal