Abstract
BACKGROUND: The cell of origin (COO) of diffuse large B cell lymphoma (DLBCL), germinal center (GC) or non-germinal center (NGC), may have prognostic significance for treatment outcome in first-line and relapsed settings (Lenz et al NEJM 2008; Thieblemont et al JCO 2011). "Double hit" DLBCL (DHL), defined by chromosomal breakpoints affecting the MYC/8q24 locus and BCL2/18q21 and/or BCL6/3q27 loci and arising either from transformation of follicular lymphoma (tFL) or de novo, has no standard effective therapy in the relapsed setting. Since new therapies are needed for poor prognostic groups of relapsed DLBCL patients (pts), we examined the efficacy of treatment with autologous T cells genetically modified to express a chimeric antigen receptor consisting of an external anti-CD19 single chain murine antibody domain with CD3ζ and 4-1BB signaling domains (CTL019 cells) in pts with relapsed/refractory GC and NGC DLBCL, DHL, and tFL as part of an ongoing phase IIa clinical trial (NCT02030834).
METHODS: Eligible pts had CD19+ DLBCL with measurable residual disease after primary and salvage therapies, were not eligible for autologous stem cell transplant (ASCT) or had relapsed/residual disease after ASCT, and had responsive or stable disease with most recent therapy. COO of DLBCL was defined immunohistochemically using the Hans algorithm (Hans et al Blood 2004) and included the DLBCL subtypes DHL and tFL that met this definition. Fluorescence in-situ hybridization was performed on diagnostic tissue using Vysis MYC (8q24), BCL2 (18q21) and BCL6 (3q27) break apart probes to determine DHL. DHL was defined by a MYC locus chromosomal translocation with a second translocation involving either BCL2 or BCL6. After steady state apheresis to collect peripheral blood leukocytes, pts received lymphodepleting chemotherapy based on clinical features and past therapies. 1 to 4 days after chemotherapy, pts received a single dose of CTL019 cells. Following CTL019, pts received no further therapy for DLBCL. Initial tumor response assessment was performed 3 months (mo) after CTL019 using IWG criteria. Enrollment started in Feb 2014; data are reported through 24 Jul 2016.
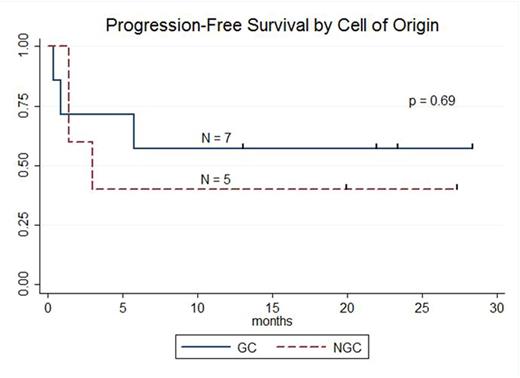
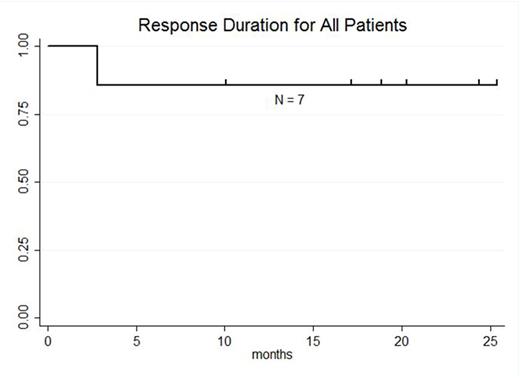
RESULTS: 13 pts with DLBCL are enrolled and evaluable for response (7 pts GC, 5 pts NGC, 1 undetermined). The median age is 59 years (range: 25-77), male:female ratio 10:3, median number of prior therapies 5 (range: 2-8), and number of pts with prior transplant 7 (54%). At enrollment, Ann Arbor stages were: Stage IV 8 pts (61%), Stage III 1 pt (8%), and Stage II 3 pts (23%) Stage IE 1 pt (8%); 3 pts (23%) had bone marrow involvement. LDH was increased in 8 pts (62%). ECOG PS was 0 in 4 pts (31%) and 1 in 9 pts (69%).Lymphodepleting chemotherapy regimens were bendamustine (90 mg/m2 x 2; 1 pt), cyclophosphamide (1 gm/m2; 2 pts), radiation-cyclophosphamide (4,000cGy-750 mg/m2; 1 pt), modified-EPOCH (3 pts), and hyper-fractionated cyclophosphamide (300 mg/m2 q12 hours x 6; 6 pts). 12 pts received 5.00E+08 (5.10 - 6.75E+06 cells/kg) CTL019 cells; 1 pt received 1.93E+08 (3.10E+06 cells/kg).Median peak CTL019 cell expansion in blood occurred 7 days after infusion (range: 2-28 days); there is no difference in peak expansion between responders and non-responders or pts with GC or NGC DLBCL.Cytokine release syndrome occurred in 9 pts (8 grade 2; 1 grade 3) and did not predict response. Transient neurotoxicity included delirium in 2/13 pts (1 grade 2; 1 grade 3) and cognitive disturbance in 1/13 pts (1 grade 1). At 3 mo post CTL019, overall response rate (ORR) is 52% (7/13 pts); ORR at 3 mo for GC 71% (5/7 pts) and NGC 40% (2/5 pts). Complete response rate (CR) at 3 mo is 38% (5/13 pts); CR at 3 mo for GC 43% (3/7 pts) and NGC 40% (2/5 pts). Best response for all pts is CR in 6 of 13 pts (46%); CR for GC 57% (4/7 pts) and NGC 40% (2/5 pts). 3 of 7 pts with GC DLBCL had tFL and all 3 achieved CR; 2 of 7 pts with GC DLBCL had DHL and both achieved CR. To date, no pt achieving CR has relapsed. Median progression-free survival is 5.8 mo for all pts, 3.0 mo for NGC pts, and not reached for GC pts (57.1% [95%CI: 17.2%-83.7%] progression-free at median follow-up 21.9 mo). At median follow-up 23.3 mo for responding pts, 85.7% [95%CI: 33.7-97.9%] maintain response.
CONCLUSIONS: A single treatment with CTL019 cells can achieve durable remissions in pts with relapsed/refractory GC and NGC DLBCL, DHL and transformed FL.
Schuster:Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Research Funding; Genentech: Consultancy, Honoraria; Merck: Research Funding; Janssen Research & Development: Research Funding; Hoffman-LaRoche: Research Funding; Gilead: Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Svoboda:Pharmacyclics: Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding. Nasta:Millennium Pharmaceuticals: Research Funding. Porter:Genentech/Roche: Employment; Genentech/Roche: Equity Ownership; Incyte: Honoraria; Novartis: Consultancy; Novartis: Research Funding; University of Pennsylvania: Patents & Royalties; Immunovative Therapies: Other: Travel, Accommodations, Expenses. Mato:ProNAi: Research Funding; TG Therapeutics: Research Funding; Acerta Pharma: Research Funding; Theradex: Research Funding; Gilead Sciences: Research Funding; Abbvie: Research Funding; TG Therapeutics: Consultancy; Pharmacyclics: Consultancy; Gilead Sciences: Consultancy; Abbvie: Consultancy. Wasik:Gilead Sciences: Equity Ownership; Seattle Genetics: Honoraria; Novartis: Research Funding; University of Pennsylvania: Patents & Royalties: NPM-ALK as an omncogene; University of Pennsylvania: Patents & Royalties: CAR T-cells; Gilead Sciences: Research Funding; Pharmacyclics: Research Funding. Lacey:Novartis: Research Funding. Melenhorst:Novartis: Patents & Royalties: Novartis, Research Funding. Chew:Novartis: Research Funding. Hasskarl:Novartis: Employment. Marcucci:Novartis: Research Funding. Levine:GE Healthcare Bio-Sciences: Consultancy; Novartis: Patents & Royalties, Research Funding. June:Pfizer: Honoraria; Johnson & Johnson: Research Funding; University of Pennsylvania: Patents & Royalties; Tmunity: Equity Ownership, Other: Founder, stockholder ; Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; Celldex: Consultancy, Equity Ownership; Immune Design: Consultancy, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal