Abstract
Introduction: The treatment of advanced Hodgkin lymphoma (HL) has undergone many changes in recent years. The original International Prognostic Score (IPS) introduced by Hasenclever et al (1998) was based on a cohort of 1618 patients (pts) mainly treated with MOPP-ABV or ABVD between the years 1983 and 1992. One third (33%) of those pts also underwent involved field radiation therapy (IFRT). Staging was based on CT. The 5-y freedom from progression (FFP) results ranged between 84% and 42%, depending on the presence of 0-≥5 risk factors. The IPS has been validated in a number of studies and settings. Currently, staging is based on baseline PET/CT, which upgrades the disease stage in 15% and downgrades it in 5% of pts compared to CT scan. Positive interim PET/CT (PET-2) is predictive of an inferior prognosis in pts with early and advanced HL. Based on these findings, several studies have used a positive PET-2 as an indication for therapy escalation. However, the majority of relapses still occur in pts with negative PET-2, highlighting the need for additional prognostic markers. We evaluated baseline factors and lab results in a prospectively collected database of pts enrolled in a multicenter clinical trial (NCT00392314) where treatment was modified based on PET-2 results. The current study aimed to develop a model for refined prediction of HL progression risk in advance-disease pts with negative PET-2, using baseline risk factors.
Methods: Pts with advanced classic Hodgkin lymphoma (HL) were stratified according to their baseline IPS; treatment was modified according to PET-2 results. Pts with IPS 0-2 initially received 2 ABVD cycles and those with IPS ≥ 3 received 2 cycles of escalated BEACOPP (EB). In the former group, if PET-2 was positive without evidence of disease progression, therapy was escalated to EB with involved site radiation therapy (ISRT) given to residual masses. If PET-2 was negative in both IPS strata, 4 additional ABVD cycles were administered. We evaluated whether traditional IPS parameters continued to predict relapse when treatment was modified based on PET-2 results, and assessed the need for new cutoffs based on ROC curves using univariate and multivariate Cox proportional hazard models.
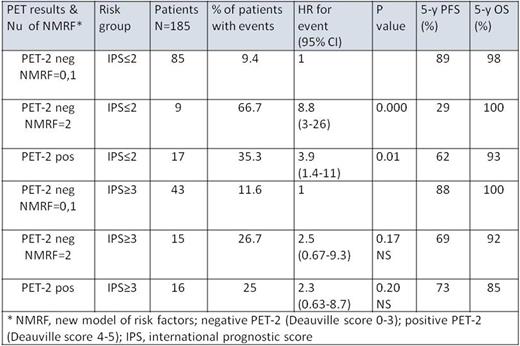
Results: Of 185 enrolled pts, 33 (18%) experienced disease progression and 27 (15%) had a positive PET-2. The 5-y PFS for the whole group, PET-2 negative and PET-2 positive subgroups was 80%, 82% and 68%, respectively (p=0.07). Eight of 33 (28%) relapsed pts had a positive Deauville score (≥3). On univariate analysis of traditional IPS parameters, male gender, age >45, stage IV disease were not found to be associated with a significantly increased hazard ratio (HR) for progression. New cutoffs for lymphocyte count and albumin level predictive of relapse were determined. The following parameters were significantly (P<0.006) associated with lower PFS on univariate analysis: albumin <3.5gm/dL, hemoglobin <10.5 gm/dL, lymphocyte count <1400 or <11% of white blood cell (WBC) count. On multivariate analysis, albumin <3.5gm/dL, lymphocyte count <1400 or <11% of WBC remained significantly associated with relapse, with adjusted HRs of 2.2 (95% CI 1.1-4.5; P<0.03) and 2.6 (95% CI 1.1-6.2; P<0.025), respectively. Hence, a score was constructed using 0, 1 or 2 of these factors. Pts with 0-1 or 2 new risk factors and negative PET-2 had a relapse rate (RR) of 10% and 42%, respectively (HR=4.7; 95% CI 4.7-11), while in pts with positive PET-2 the RR was 30% (Table 1). However, one should bear in mind that pts with IPS 0-2 initiated therapy with ABVDx2 and those with IPS≥3 initiated therapy with EBx2.
Conclusions: The current analysis, using data prospectively collected during a phase II study but analyzed retrospectively after pts had been stratified by IPS, suggests a scoring model that could refine the IPS-based identification of pts at a higher risk for disease progression, even if their PET-2 is negative. The findings imply that pts with 2 new risk factors (19% of pts) may need to initiate more intensive therapy than ABVD. However, given the retrospective nature of the analysis, its results should be interpreted with caution. The model needs to be verified in an independent cohort of pts with advanced HL using uniform treatment protocols.
Bairey:Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal