Abstract
Introduction: Prior studies have shown preliminary clinical efficacy in combining CpG-ODN with radiation therapy (XRT) to patients with indolent B-cell lymphoma. We report interim Phase 1/2 data of combination XRT and SD-101, a synthetic class C CpG-ODN TLR9 agonist, selected for the strong induction of type I interferon.
Methods: This dose-escalation Phase 1/2 trial enrolled patients with untreated indolent B-cell lymphoma having at least 2 measurable lesions (one palpable). The primary endpoints were safety and alpha-interferon-gene induction. Secondary endpoints included efficacy assessment using Cheson (1999) criteria and quantification of changes in tumor-infiltrating lymphocytes. A single lesion was treated with XRT (2 Gy daily X 2 days) followed by a single intratumoral dose of SD-101 (same lesion). 4 additional doses of intratumoral SD-101 were given weekly over next 4 weeks. The treated lesion and distal lesions were monitored during the study. Pharmacodynamic assessment included flow cytometry analysis of immune infiltrates in an FNA sample of the treated tumor and RT-PCR RNA assay of whole blood to assess induction of alpha-interferon genes. Efficacy assessment included imaging (CT at 3, 6, and every 6 months thereafter).
Results: As of 01 Aug 2016, 13 patients were treated with escalating doses of SD-101 at 1, 2, 4 or 8 mg/dose. There were no dose limiting toxicities. The majority of related adverse events (AEs) were Grade 1 (mild). The most common were flu-like symptoms (chills, headache, malaise, myalgia), typically resolving within ≤ 48 hours without requiring intervention (e.g., acetaminophen). An induction of alpha-interferon genes occurred at all dose levels with a similar level of induction.
For the dose expansion phase, 15 patients were enrolled at 1 mg (6) and 8 mg dose group (9). Similar to dose escalation phase, the most common related events were again flu-like symptoms, typically resolving within ≤ 48 hours mitigated with acetaminophen. There was one dose delay for Grade 3 neutropenia in one patient (1 mg) that resolved following drug interruption. Twenty-nine Grade 3 transient flu-like symptoms were reported for four patients (44%) who received the 8 mg dose (e.g., chills headache, malaise, myalgia, and fatigue). Only one of the four patients experienced all 5 Grade 3 flu-like symptoms after intratumoral injection. There were no grade 4 or serious events reported, and no patient discontinued due to an adverse event.
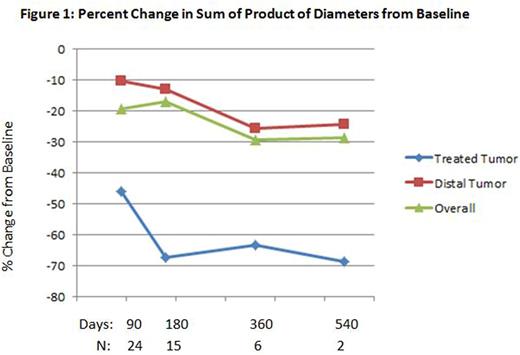
A reduction in tumor sizes was observed in the study over time (see Figure 1). At Day 90 the median changes in the product of diameters from baseline were -46.1% and -10.2% for treated tumor and distal tumors, respectively. At Day 540, the median changes were -68.6% and -24.1%, respectively.
In patients with follicular lymphoma (largest subgroup), preliminary data suggest a higher percentage of CD8+ T cells in the treated lesion (FNA at day 8) correlated with an increased abscopal response.
Conclusions: Intratumoral SD-101 following radiation therapy has been well tolerated and preliminary efficacy, promising. Abscopal anti-tumor activity was observed with preliminary data suggesting that a higher percentage of CD8+ T cells in the treated lesion correlated with an increased abscopal response seen in follicular lymphoma. Enrollment is ongoing with an option for cycle 2 retreatment.
Levy:Kite Pharma: Consultancy; Five Prime Therapeutics: Consultancy; Innate Pharma: Consultancy; Beigene: Consultancy; Corvus: Consultancy; Dynavax: Research Funding; Pharmacyclics: Research Funding. Reagan:Seattle Genetics: Research Funding. Friedberg:Bayer: Other: Data Safety Monitoring Committee. Bartlett:Gilead: Consultancy. Leung:Dynavax: Employment. Peterkin:Dynavax: Consultancy. Xing:Dynavax: Employment. Coffman:Dynavax: Employment. Janssen:Dynavax: Employment. Candia:Dynavax: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal