Abstract
Background: Rituximab, administered intravenously (IV), is the mainstay of treatment for CD20+ B-cell non-Hodgkin lymphoma (NHL). A subcutaneous (SC) formulation has been approved in Europe and other countries based on the SABRINA study, which showed non-inferior pharmacokinetics and comparable safety and efficacy for rituximab SC compared with IV in treatment-naïve patients with follicular lymphoma (FL) (Davies A, et al. Lancet Oncol 2014;15:343-52). Furthermore, rituximab SC reduces healthcare resource burden (De Cock et al. PLoS One 2016;11:eD157957) and improves patient satisfaction and convenience compared with IV (Rummel et al. Blood 2015;18:A469). MabRella is a global umbrella study comprising three local open-label, single-arm, Phase 3b studies evaluating the safety of first-line (1L) rituximab SC treatment in patients with FL or diffuse large B-cell lymphoma (DLBCL) with a focus on administration-related reactions (ARRs). The studies share a core protocol, study endpoints, and timepoints, but have flexibility for modifications at local level. Data from participating countries is pooled for pre-defined global analyses.
Methods: Patients were aged 18-80 years with grade 1-3a FL or DLBCL, and ECOG PS of ≤3. All patients had received ≥1 full dose of rituximab IV as 1L induction or maintenance prior to study entry, and were eligible to receive at least 4 additional cycles of induction or 6 additional cycles of maintenance. Patients with FL or DLBCL receiving induction therapy were treated with rituximab SC 1400mg every cycle (14, 21, or 28 days) for 4-7 cycles, in combination with standard chemotherapy. FL patients undergoing maintenance treatment received single-agent rituximab SC 1400mg every 2 months for 6-12 cycles. The primary endpoint was incidence of ARRs following multiple doses of rituximab SC; ARRs were defined as all adverse events (AEs) occurring within 24 hours of rituximab administration and considered related to the study drug by the investigator. Secondary safety endpoints included grade ≥3 AEs and serious AEs (SAEs). The safety analysis included all patients who received ≥1 dose of study treatment. Safety data were not collected for rituximab IV, as patients entered the trial after they had switched to SC. Data are presented from a planned interim analysis of safety (cut-off March 31, 2016).
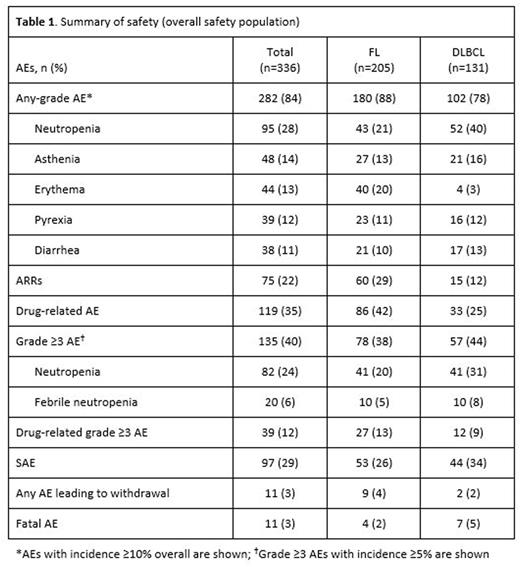
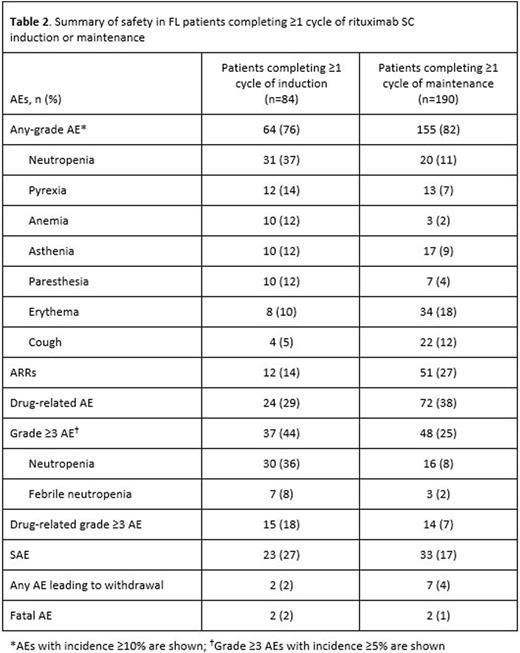
Results: At the data cut-off, the safety population comprised 336 patients: 157 from Italy; 139 from Spain; and 40 from North Africa (Tunisia and Algeria). The median (range) age was 59 (19-80) years and 50% patients were male; 205 patients had FL and 131 had DLBCL. With respect to patients with FL, 84 completed ≥1 cycle of rituximab SC induction (37 completed all 7 cycles) and 190 completed ≥1 cycle of maintenance (169 completed 6 cycles and 26 completed all 12 cycles). Among DLBCL patients, 37 completed all 7 cycles of induction. Overall, 282 patients (84%) experienced an AE during treatment (Table 1). ARRs were observed in 75 patients (22%), the most common of which (≥2% incidence) were erythema (12%) and injection-site erythema (4%). Other AEs reported in ≥10% of patients were neutropenia, asthenia, erythema, pyrexia, and diarrhea (Table 1). Grade ≥3 AEs occurring in ≥5% of patients were neutropenia (24%) and febrile neutropenia (6%). Grade ≥3 ARRs were observed in 4 patients (1%). SAEs were reported in 29% of patients (8% drug-related). Of 11 deaths attributed to AEs, 2 were considered to be related to treatment (Klebsiella infection and sepsis). The safety profile of rituximab SC was generally comparable between FL and DLBCL patients, although neutropenia (any grade and grade ≥3) was more prevalent in DLBCL patients, while erythema (any grade) was more common in FL patients (Table 1). The profile of AEs was similar during induction and maintenance, but the intensity and seriousness of events was lower during maintenance (Table 2). Geographic location (Spain, Italy, North Africa) of study sites did not appear to impact on the safety profile of rituximab SC.
Conclusions: In line with previous reports (Davies et al. 2014), rituximab SC is well tolerated during the 1L treatment of patients with NHL. The safety profile of 1L rituximab SC is similar to reports for IV administration, with the exception of an expected increase in ARRs, the majority of which are mild or moderate in intensity, and resolve spontaneously.
Panizo:Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche Pharmaceuticals: Speakers Bureau. Rigoroso Mendoza:F Hoffmann La Roche Ltd: Employment. Truman:Aurinia Pharmaceuticals: Consultancy; F Hoffmann La Roche Ltd: Consultancy, Equity Ownership. Smith:F. Hoffmann La Roche Ltd.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal