Abstract
Introduction. For more than a decade it's postulated that the addition oftyrosine kinase inhibitors (TKI) to chemotherapy has dramatically improved the long term outcome in Ph-positive adult acute lymphoblastic leukemia (Ph+ ALL). Nevertheless whether do we need chemotherapy at all and if yes - how intensive it should be, is still the matter of debates. The only randomized trial addressing this issue (GRAAL, Blood 2015, 125: 3711-3719) has demonstrated the lack of benefit of more intensive induction at all checkpoints: complete remission (CR) rate, major molecular complete remission (MMolCR), molecular complete remission (MolCR), progression disease (PD) and resistance. We have conducted two consecutive trials in Ph+ ALL aiming to evaluate the efficacy of more and less intensive chemotherapy approach in combination with constant non-stop 600 mg Imatinib application.
Aim. Toanalyze of the protocol RALL-2009 with ITK and RALL-Ph+-2012 effectiveness in patients with Ph+ ALL. The primary objective was the major molecular complete remission (MMolCR) rate after induction (70th day), patients being then eligible for allogeneic stem cell transplantation (SCT) if they had a donor, or autologous SCT if in MMolR and no donor.
Patients and methods. Since Jan 2010 till July 2016, 120 new cases of ALL were verified in our National Research Center for Hematology with 68 (56,7%) of them being B-cell precursors ALL and 25 diagnosed as Ph-positive (36,8%). Since 2010 till 2012, 10 Ph+ ALL pts (median age 35 years (19-68), m/f (50%)/(50%), CNS disease=1, WBC> 30*109/l=5(50%), bcr/abl transcript p190/p210/p190+210 6(60%)/3(30%)/1(10%)) were treated according to RALL-2009 protocol (ClinicalTrials.gov public site; NCT01193933) with parallel Imatinib. This protocol includes 8 cytostatic drugs and no intervals between treatment phases. Since 2012 till now 15 other pts (median age 40 years (17-61); m/f 7(46,7%)/8(53,3%); CNS disease=1, WBC>30*109/l=5(33,3%), bcr/abl transcript p190/p210/p190+210 9(60%)/5(33%)/1(7%)) were included in RALL- Ph+-2012 protocol, based mainly on 600 mg Imatinib with prednisolone, VNCR, L-asp, followed by 6-MP and MTX. Both protocols suggested the shift to Dasatinib (100-140 mg) after non-achievement of MolCR at day 70 of treatment. MolCR was stated if no bcr/abl chimeric transcript was detected by PCR with 10-4 sensitivity. All patients were considered as candidates for allogeneic HSCT if HLA-identical donor was available.
Results. At day 70th disregarding the chemotherapy intensity there was 40% of MolCR on both protocols (RALL-2009 - n=4 and RALL-2012 - n=6). No death within 2 months of induction/consolidation were registered on less intensive protocol in comparison with 2 cases on RALL-2009. Hematological CR was achieved in all pts (except two early deaths on RALL-2009) - 23 of 25 (92%). There was one autologous HSCT in older pts, included in RALL-2012 (n=3, aGVHD and severe infections, at a median +4 months after HSCT and more than 12 months of CR duration).
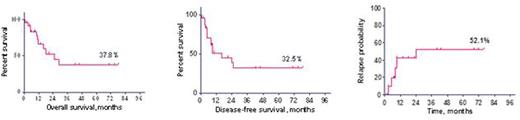
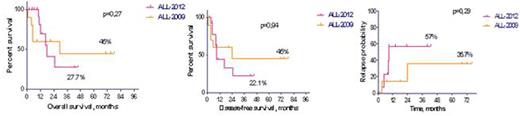
The 3y OS, DFS and relapse probability (RP) for all 25 pts constituted 37,8%, 32,5% and 52,1% (Fig. 1). The long-term outcome on both protocols (RALL-2009 and RALL-2012) was similar: OS - 45% vs 27,7% (p=0,27), DFS - 45% vs 22,1% (p=0,94), RP - 35,7% vs 57% (p=0,29), respectively (Fig.2).
Conclusion. De-intensification of the chemotherapy does not affect the effectiveness of the therapy Ph-positive acute lymphocytic leukemia in era of the tyrosine kinase inhibitors. We haven't seen differences in achievement of molecular remission when we deescalated chemotherapy (40% vs. 40%). However, when we reduced toxicity of the chemotherapy in ALL-2012 protocol, we were able to realize more extra allo-BMT and it could improve long-term results of the therapy Ph+ ALL.
Overall, disease-free survival and relapse probability in patients with Ph+ ALL on RALL protocols.
Overall, disease-free survival and relapse probability in patients with Ph+ ALL on RALL protocols.
Overall, disease-free survival and relapse probability in patients with Ph+ ALL on RALL-2009 and RALL-2012 protocols.
Overall, disease-free survival and relapse probability in patients with Ph+ ALL on RALL-2009 and RALL-2012 protocols.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal