Abstract
Introduction: There is a paucity of data on the distribution of acute leukemia (AL) across ethnic groups. Historically, B-cell acute lymphoblastic leukemia (B-ALL) and acutepromyelocytic leukemia (APL) are over-represented in Hispanic patients, while acute myeloid leukemia (AML) is less frequent. We analyzed the ethnic distribution of AL in Florida based on incidence rates and nativity differences using Florida Cancer Data System (FCDS). In our study, B-ALL and APL were more common in Hispanics vs. non-Hispanics (IRR of 1.627 and 1.302, respectively); however, contrary to prior reports, AML was also more common (IRR 1.533) (Swords et al, BCJ 2016, in press). No nativity differences were observed. Given this novel finding of increased AML incidence in Hispanics in South Florida, we examined AML patients at Sylvester Comprehensive Cancer Center (SCCC) to look for ethnic differences in mutational frequency.
Methods: We obtained molecular genetic data on AML patients treated from 2012-2015 across multiple centers.Genomic DNA from bone marrow or peripheral blood was sequenced for ASXL1, DNMT3A, FLT3 TKD, IDH1, IDH2, KIT, NPM1, PHF6, TET2 on the IlluminaMiSeq platform using a lower limit of detection of 5% and minimum coverage of 500X. Alignment and variant calling were performed usingNextGENe® software as previously described byGenoptix, Inc. MLL PTD, FLT3 ITD and CEBPA mutations were detected by PCR amplification followed by fragment analysis.For patients treated at SCCC, we obtained IRB approval to conduct retrospective chart reviews for clinical and pathologic data. Categorical data was analyzed by Fisher exact test or Chi-square test as appropriate. Continuous data was analyzed by independent t-test and Wilcoxon-Mann Whitney test for parametric and non-parametric data, respectively. Statistical analysis was performed using Stata (version 13.0).Circos plots were created usingCircos.
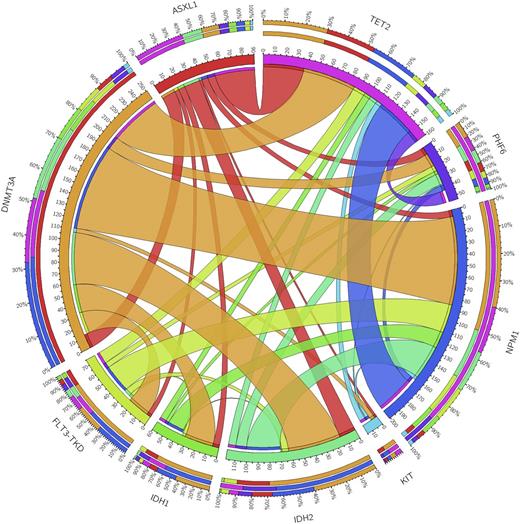
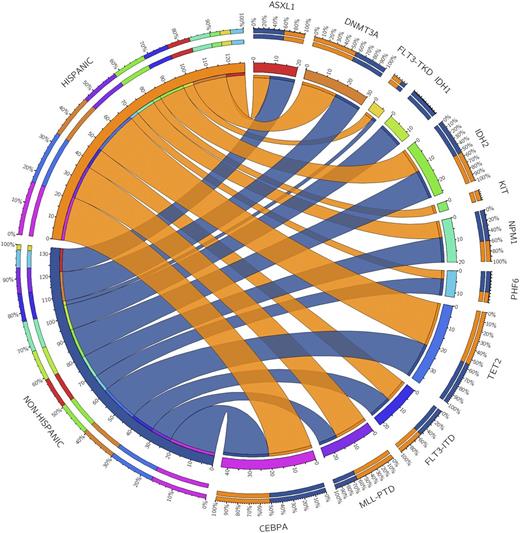
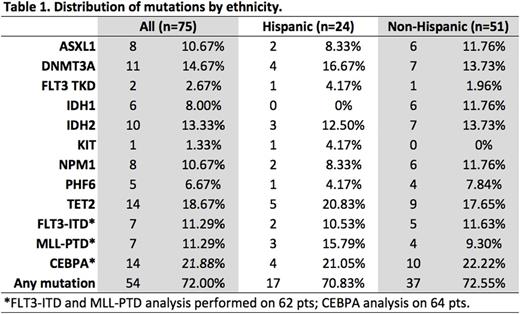
Results: We examined the 11-gene mutational profile of 927 unique AML patients. The frequency of these mutations was consistent with previous reports (Park et al, NEJM 2012). DNMT3A, NPM1, and FLT3 were the most common abnormalities (Figure 1A). 75 of these 927 patients were treated at our center. Of these, median age was 60.2 years; 53.3% were female; 80% were white, 13.3% black,6.7% other. 68% (n=51) self-identified as non-Hispanic and 32% (n=24) as Hispanic (Table 1). Median WBC was 3.95 K/µL (1.4-100.7); 37% had antecedent MDS or MDS-related changes; 8% had favorable-, 69% intermediate-, and 23% poor-risk cytogenetics. There were no differences in WBC, MDS, or cytogenetic risk by ethnicity. In the 75 patient SCCC cohort, TET2 (n=14, 18.7%), CEBPA (n=14, 21.9%), and DNMT3A (n=11, 14.7%) were the most common mutations. 16 patients had an IDH mutation (10 IDH1 and 6 IDH2). We found no statistically significant difference in mutation frequency in Hispanic vs. non-Hispanic patients (Figure 1B); however, there was a non-significant increase in MLL-PTD mutations in Hispanic patients (15.8% vs. 9.3%, p=0.665) and IDH1 mutations in non-Hispanics (11.8% vs. 0%, p=0.168).
Conclusions: We present one of the largest examinations to date (n=927 patients) of mutational frequency in AML. Our findings corroborate prior literature; however, we did note the rare co-occurrence of TET2 and IDH mutations-showing the 2 are not always mutually exclusive. We examined demographic data on a subset of these patients treated at SCCC, where we have a large Hispanic population, and assessed for ethnic differences in mutational frequency. We found a suggestion of increased MLL PTD mutations, a poor-risk abnormality, in Hispanic patients, and increased IDH1 mutations in non-Hispanics, but found no significant differences. This may be due to our relatively small sample size. We are now examining the full 927 patient cohort for demographic data and updated results will be presented. If certain AML mutations cluster by ethnicity, this might explain differing incidence rates and outcomes in Hispanics, and examination of predisposing heritable or environmental factors should be pursued.
Table 1. Distribution of mutations in an 11-gene AML molecular profile by ethnicity in 75 patients with AML.
Figure 1.Circosplots characterizing the distribution of concomitant mutations in 927 patients with AML (A); and the incidence of mutations by ethnicity in 75 patients with AML (B).
Vaupel:Genoptix, a Novartis Company: Employment. Hall:Genoptix, a Novartis Company: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal