Abstract
Introduction: The Children's Oncology Group (COG) conducted a randomized, Phase III study evaluating Gemtuzumab Ozogamicin (GO), a humanized anti-CD33 antibody, for children with de novo AML. This analysis describes longer term outcomes for patients assigned to GO as well as relapse risk factors and subsequent outcome for patients experiencing relapse after AAML0531 therapy.
Methods: AAML0531 enrolled 1,022 evaluable patients ages 0-29 to receive either standard five-course chemotherapy with or without 2 doses of GO. All high risk and those intermediate risk patients with family donors received stem cell transplant rather than the last 2 chemotherapy cycles and the 2nd GO dose. Analysis of characteristics impacting cumulative incidence to relapse and overall survival from relapse were performed (data cutoff 3/31/16).
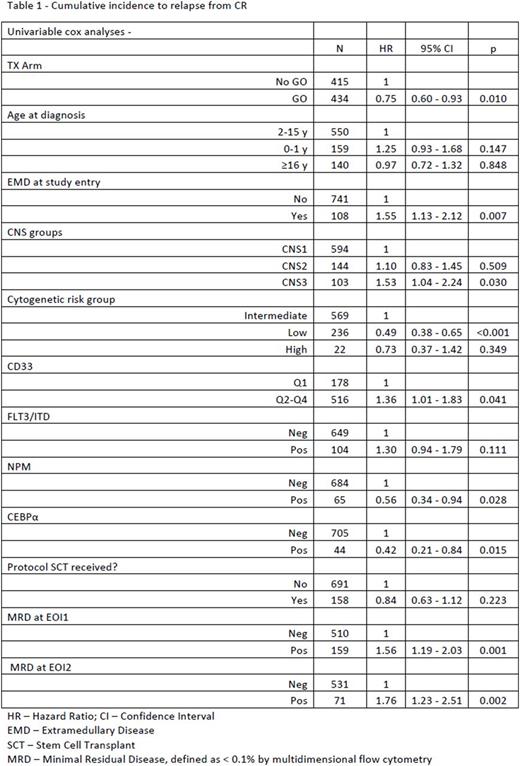
Results: Updated outcome analyses demonstrated GO improved 5 year (yr) event-free survival (EFS) of 51.4 ± 4.5% vs 48.5 ± 3.2% p=0.055, but no benefit in 5 yr overall survival (OS), 64.9 ± 4.4% v 64.1 ± 3.1% p=0.406. A 5-yr cumulative incidence to relapse (REL) from complete remission (CR) (N= 849) was seen in 37.8% of patients, with significantly less among those receiving GO vs no-GO (33.6% vs 42.2% p=0.01). In Univariate analysis, children with NPM and CEPBA mutations, low risk cytogenetics, and higher CD33 expression had lower relapse risk (RR). Those with extramedullary disease (EMD) at diagnosis, FLT3/ITD mutations, CNS3 disease, and minimal residual disease at end of induction 1 and 2 predicted higher RR (Table 1). Multivariate analysis determined that EMD at diagnosis (HR 1.68, 95% CI 1.10-2.56, p=0.016) and minimal residual disease (MRD) at end of induction 1 (EOI1) (HR 1.61, 95% CI 1.13-2.30, p= 0.008) predicted significantly higher RR, while low risk cytogenetics predicted lower RR (HR 0.48 95% CI 0.33-0.70, p<0.001). The 5 yr OS from REL (n=318) for all patients on study was 32.5% with no advantage to those receiving GO in de novo therapy (30.0 ± 8.1% vs 34.4 ± 7.3% no-GO, p=NS). GO did have impact on 180 day OS from REL (74 ± 6.3% v 64 ± 6.2% p= 0.018), however, impact was lost by 1yr ( GO-52 ± 7.3% v No GO 51.6 ± 6.5%). Among all patients, those with REL <180 day from first CR had dismal prognosis, while those with REL > 1 yr from first CR had significantly better 5-yr OS after REL (Table 2).
Conclusions: AAML0531 previously reported GO significantly improves EFS by reducing RR when given to children with de novo AML. This cumulative analysis updates prognostic factors influencing RR and subsequent impact upon OS after REL. EMD at diagnosis and MRD at EOI1 are significantly predictive of relapse risk in multivariate analysis while those with low risk cytogenetics have significantly lower incidence of relapse. While GO significantly improves the 5 yr incidence of relapse from first remission, it does not impact 5-yr OS after first relapse. GO does improve 180-day OS after first relapse, but this impact loses significance at 1 yr post REL. Overall survival is significantly impacted by the length of 1st CR, with worst prognosis for those relapsing within 180 days. Work is ongoing to define other risk factors for altered OS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal