Abstract
Introduction
Severe aplastic anemia (SAA) is an immune mediated bone marrow failure syndrome characterized, among other features, by reduced numbers and dysfunctional regulatory T cells, as shown by our group. Using mass cytometry, we identified an immune signature that predicts clinical response toimmunosuppression (IST)consisting of two Treg subsets (A and B), differentially expressing CD45RA, CD95, CD7, CD28, and CCR4(Kordasti Blood 2016). Treg B are more suppressive and enriched in cell cycle related proteins, suggesting their proliferative potential. The aim of this study was to investigate the in vitro expandability of Treg subpopulations as well as their function, stability, plasticity, clonality and gene signature following expansion with the aim of using autologous in vitro expanded Tregs as a therapeutic option for AA patient.
Methods and results
Treg expansion, phenotype, and function
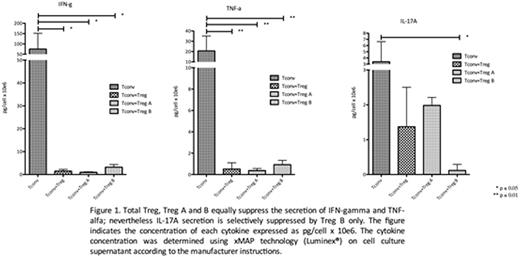
Total Tregs from AA and HDs are sensitive to low dose IL-2 as shown by STAT5 phosphorylation (p < 0.001, n = 6 AA and 2 HDs). Moreover Tregs from AA and HDs can be expanded at a comparable rate (fold increase 33x vs 21x, p = n.s. n = 6 AA and 8 HDs) in a Treg promoting culture (stimulation with anti CD3/CD28 beads and IL-2 1,000 IU/ml for 4 weeks with all-trans retinoic acid and rapamycin). The expanded total Tregs have the phenotype of Treg B. To investigate the potential differences between Treg A and B in terms of function and expandability, Treg A (CD4+CD25hiCD127loCD45RAhiCD95-CCR4lo) and B (CD4+CD25hiCD127loCD45RAloCD95+CCR4hi) were sorted from 6 HDs and expanded for 4 weeks (as above). On average Treg A and B expanded equally (Treg A 45x, and B 33x fold increase, p = n.s.). After expansion Treg A up-regulate CD95 expression (p = 0.005) similar to Treg B phenotype. Expanded Treg A and B both suppress proliferation of autologous Tconv (n = 3, p < 0.001) as well as the IFN-g and TNF-a secretion. Only Treg B suppressed IL-17A secretion (n = 3, p < 0.05) (figure 1).
Treg-specific demethylated region (TSDR)
Treg stability was assessed by DNA methylation analysis by deep ampliconbisulphitesequencing of the TSDR. TSDR CpG sites in expanded HDs and AA Tregs are > 98% unmethylated, confirming their stability. Interestingly, TSDR sites were less methylated in Treg B compared to Treg A (5% v 35%), but it decreases to a similar level as Treg B after expansion.
Treg plasticity after expansion
To investigate the plasticity, total Tregs, Treg A, and Treg B were cultured for 5 days in a Th17 polarizing culture (containing IL-1b,IL-6, IL-2). After 5 days of culture, only total Tregs were secreting IL-17A (p = 0.013), but neither Treg A, nor Treg B. Despite IL-17A secretion, Tregs were still able to suppress autologous Tconv proliferation (n = 3, p= 0.001).
T cell receptor diversity
To track the clonal origin of Treg A and B, we performed amplification and sequencing of CDR3 using immunoSEQ Platform, as already described. Our results show that both AA and HDs expanded Tregs show a comparable level of TCR VbCDR3 diversity (average clonality score 0.12); moreover Tregs, Treg A, and Treg B are polyclonal, pre and post expansion (clonality score 0.01 for all the subsets). Finally, Treg A and B do not share any TCR sequence and do not have any overlap in their TCR repertoire, suggesting they originate from different clones.
Gene expression
Expanded Tregs (total, A and B) were compared to established human Treg gene signature (Ferraro PNAS 2014). Treg B aremore enriched in Treg genes compared Treg A (Tregs B vs A false discovery rate (FDR) < 0.0001). IL-7 and CD80 are among the top 5 expressed genes in all 3 subpopulations, which are important for Tregs' motility and homeostasis.
Conclusions
In summary, AA Tregs can be expanded in vitro and expanded Tregs are more similar to Treg B, which are lacking in SAA as shown before. Although we were not able to sort Treg subpopulations from AA patients due to very low numbers, the expanded Treg A and B from HDs demonstrate a functional Treg profile with minimal plasticity. Interestingly the main difference between Treg A and B is the ability of Treg B to suppress IL-17A secretion. This is important, as IL-17 plays a crucial role in AA pathophysiology (Peffault de Latour Blood 2010). Overall, the current study suggests that in vitro expansion of AA Tregs is feasible. The expanded Tregs show the phenotype of the most functional Treg subset and likely to control the inflammatory response inAA, hence pave the way toward a novel cellular therapy for SAA.
Marsh:Alexion pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal