Abstract
Background: Platelets are a critical component of primary and secondary hemostasis and clinically important in bleeding and thrombocytopenic patients. However, they remain logistically problematic in blood banking due to their short shelf life, complicated pooling process, high wastage rate and cost, and high rate of transfusion reaction. In vitro studies have shown that stored platelets exhibit minimal (if any) platelet aggregation, and the clinical presumption is that they retrieve their function post-transfusion in vivo. In addition, stored platelets have been shown to have increased levels of platelet microparticles (PMP) that are directly correlated to the length of the storage period. Clinical findings have revealed that patients with thrombocytopenic disorders such as HIT, ITP, TTP, SCD, arterial thrombosis, and certain types of cancer are also associated with increased levels of PMP, which may contribute to bleeding prevention and subsequent thrombosis in these patients. We hypothesized that the same principle may be attributed to stored platelets, and that the retrieved platelet function after transfusion may be due to the procoagulant properties of PMP. Here we evaluated a newly developed, freshly prepared PMP product as a possible means for topical and/or IV application in bleeding and/or thrombocytopenic patients.
Methods: Platelet rich plasma (PRP) of healthy individuals was concentrated via centrifugation at 400xg for 8 minutes and a complete blood count (CBC) was performed. Five-day old platelet units were obtained from the blood bank. Platelet counts for fresh and stored PRP were standardized at approximately 1.2x106/μL. PRP was then briefly sonicated for two-second intervals, repeated four times (VibraCell VC50 Probe Sonicator, Sonics and Materials Inc.). The sonicated platelets were then centrifuged at 18,000xg for one hour to obtain platelet microparticles (PMP), and 75% of the supernatant was removed. The pellet was re-suspended in the remaining 25% of the supernatant to obtain concentrated PMP. A thrombocytopenic model was developed by replacing PRP from a normal donor whole blood sample with autologous plasma for a final platelet count of approximately 15x103/μL. The prepared PMP was mixed 1:4 with the thrombocytopenic whole blood sample and evaluated for clot kinetics via thromboelastography (TEG) (TEG5000 Thromboelastograph¨ Hemostasis Analyzer, Haemonetics), thrombin generation assay (TGA) (Calibrated Automated Thrombogram¨, Stago), activated clotting time (ACT) (International Technidyne Corporation, Hemochron¨ Signature Elite Whole Blood Microcoagulation System), and whole blood aggregation (Chrono-Log Lumi-Aggregometer, Model 700). PMP size and concentration were also measured via NanoSight (NanoSight NS300, Malvern) and flow cytometry was used to identify and measure PMP activity (FACSCantoª II, BD Biosciences). All analyses were performed according to manufacturer protocols. The student t-test was performed in all statistical analysis.
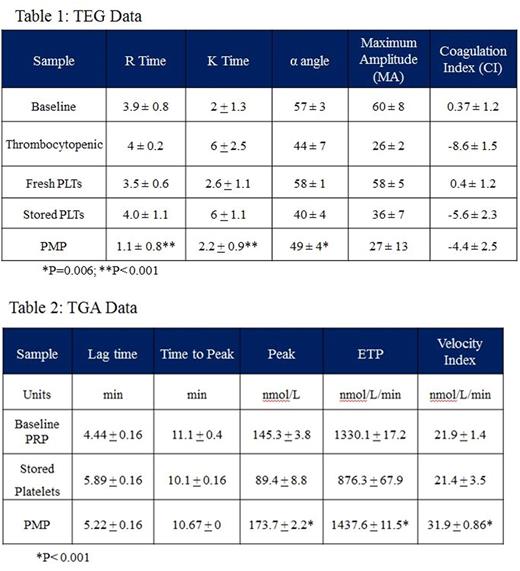
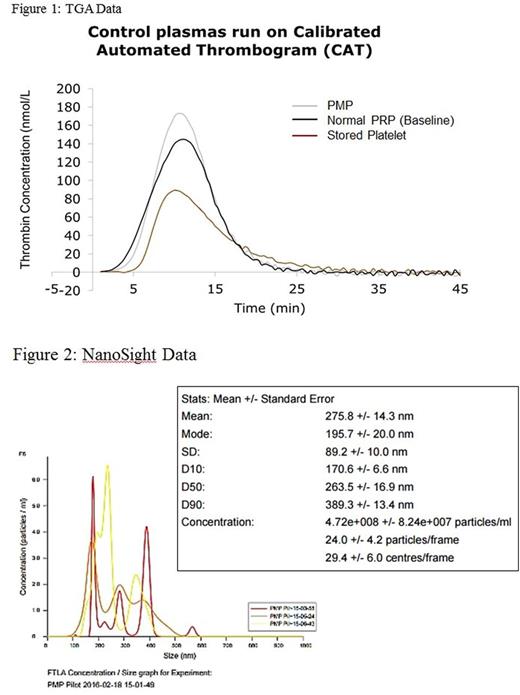
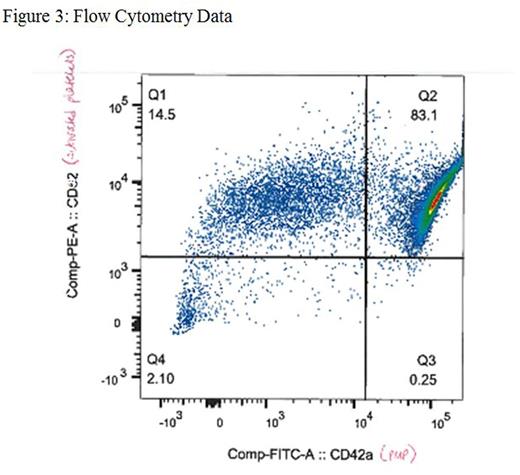
Results: TEG revealed increased coagulability of PMP as compared to baseline whole blood, thrombocytopenic whole blood, and fresh and stored PRP via decreases in R time (p<0.001), K time (p<0.001), and α angle (p=0.006) (Table 1). TGA showed increased thrombin generation of PMP compared to fresh and stored platelets through increases in the peak (p<0.001), endogenous thrombin potential (ETP) (p<0.001), and velocity index (p<0.001) (Table 2, Figure 1). Particle analysis by NanoSight revealed a PMP concentration of 4.72x108/mL (Figure 2), and flow cytometric analysis of PMP was positive for platelet activation markers CD62 and CD42a (Figure 3).
Discussion: Our study confirmed the significant procoagulant properties of our newly developed PMP product as compared to blood bank stored platelets. This PMP product may have useful clinical implications in the future that may reduce complications associated with platelet transfusions. Further in vivo studies will be conducted for evaluation of the potential for topical and IV application of PMP in thrombocytopenic mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal