
Introduction
Anti-erythroid antibodies are the classical marks of serologic and hemolytic transfusion reactions. These reactions can occur acutely or in a delayed timeframe, while the sensitizing antibody may derive from the host or be passively acquired. In those with concurrent hemolysis, the red blood cell (RBC) breakdown may be severe enough to command supportive care. However, in those with non-hemolytic delayed serologic transfusion reactions (NH-DSTRs), the threat applies more towards the future rather than the present time. Downstream hazards range from hemolytic disease of the newborn, to delays and difficulties sourcing antigen-negative blood (when the antibody is known), or an anamnestic response with higher odds of hemolysis on antigen re-exposure (when the antibody becomes unknown by evanescence and healthcare fragmentation). Data are lacking on inpatient outcomes associated with discovering a new NH-DSTR during a hospital admission. A review of NH-DSTRs was thus performed in a large academic hospital (34,000 RBC dispensations annually).
Methods
A retrospective review of a transfusion reaction database was undertaken at a large academic hospital in Toronto, Canada. Transfusion reactions (TRs) occurring during inpatient admissions (excluding emergency room and outpatient visits) from 1/1/2010-31/12/2015 were included. NH-DSTR was defined as the presence of a new antibody on repeat screen post transfusion with no evidence of hemolysis. Other anti-RBC antibody mediated TRs included acute hemolytic transfusion reactions (AHTR) (both host-derived and passively-acquired [from products such as intravenous immunoglobulin]), and delayed hemolytic transfusion reactions (DHTR) occurring with or without serologic findings. A comparison was also made against all inpatient TRs not due to RBC antibodies (non-anti-RBC TRs). Disturbances deemed unrelated to transfusion were excluded. Outcomes included length of stay (LOS), interval between TR recognition and discharge, severity of TR (as per the International Society of Blood Transfusion grading system), and death.
Results
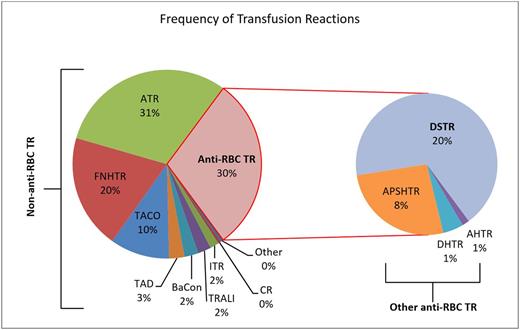
A total of 783 inpatient TRs were reviewed. The distribution of TRs (Figure 1) included 562 (71.8%) non-anti-RBC TRs and 221 (28.2%) anti-RBC TRs. Within the anti-RBC TRs, 159 (71.9%) were classified as NH-DSTRs. The mean age of all patients was 57 (± 17) with 49.4% of reactions occurring in females. Compared with non-anti-RBC and other anti-RBC transfusion reactions, NH-DSTRs were significantly less frequently classified as severe (Table 1). The overall LOS and remaining days in hospital after TR were significantly longer in those with NH-DSTRs compared with the two other groups (Table 1). Post-reaction LOS was longer by a median of 5 or 7 days for NH-DSTR versus non-anti-RBC TRs and other anti-RBC TRs respectively. There was no significant difference between groups when evaluating inpatient mortality.
Conclusions
NH-DSTRs are associated with a longer LOS when compared with all other TRs. This relationship holds even in comparisons with other anti-RBC TRs. Causality is not established by this analysis, nor is there a biologic rationale for a NH-DSTR to directly impact LOS. However, the propensity to form a new anti-RBC antibody may reflect an underlying pro-inflammatory comorbid state that itself may be influencing LOS. Further studies are needed to confirm this association. Nevertheless, given any potential for additional/current impacts beyond future ramifications, the precautionary principle is strengthened for the value of curating the full extent of a recipient's antibody history, and prophylactically matching for minor antigens if resources permit.
Comparison of outcomes between NH-DSTRs versus non-anti-RBC TRs and other-anti-RBC TRs.
Comparison of outcomes between NH-DSTRs versus non-anti-RBC TRs and other-anti-RBC TRs.
Frequency of transfusion reactions from January 1, 2010 to December 31, 2015. Abbreviations: allergic transfusion reaction (ATR), febrile non-hemolytic transfusion reaction (FNHTR), transfusion associated circulatory overload (TACO), transfusion associated dyspnea (TAD), bacterial contamination (BaCon), transfusion related acute lung injury (TRALI), inflammatory transfusion reaction (ITR), citrate reaction (CR), acute passive serologic/hemolytic transfusion reaction (APSHTR).
Frequency of transfusion reactions from January 1, 2010 to December 31, 2015. Abbreviations: allergic transfusion reaction (ATR), febrile non-hemolytic transfusion reaction (FNHTR), transfusion associated circulatory overload (TACO), transfusion associated dyspnea (TAD), bacterial contamination (BaCon), transfusion related acute lung injury (TRALI), inflammatory transfusion reaction (ITR), citrate reaction (CR), acute passive serologic/hemolytic transfusion reaction (APSHTR).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal