Background
Thrombocytopenia is common in children receiving cancer chemotherapy. In addition, children with cancer are also at increased risk of acute thrombotic events (TE). When these two complications occur simultaneously, TE management with anticoagulants (ACT) poses a unique challenge, as ACT use in thrombocytopenic patients increases their bleeding risk. Current guidelines provide only best practice recommendations on how to manage this treatment conundrum, and even these recommendations vary on optimal management due to the lack of evidence to guide treatment. To date, no systematic literature review summarizing the available paediatric data on treatment of TE in the presence of thrombocytopenia has been conducted. The aim of our systematic review was to summarize the data available to evaluate the safety of ACT for management of TE in paediatric oncology patients during periods of thrombocytopenia.
Methods
We systematically searched MEDLINE and EMBASE from the OVID platform from inception to April 15th 2016 for studies that included children aged less than 18 years with diagnosis of cancer complicated by an objectively confirmed TE, whose anticoagulation therapy was complicated by a period of thrombocytopenia (as defined by the study author). We included all study designs. Two authors (LB and AA) screened the data at title then full-text level to select eligible studies. Disputes were arbitrated by a third author (UA) until a consensus was reached. Our primary outcome was haemorrhagic complications, categorized as minor or major according to paediatric ISTH criteria. Bleeding episodes were divided according to anticoagulation intensity (age-appropriate, agent-specific full dose vs. half-dose, as per CHEST guidelines) and degree of thrombocytopenia (i.e. severe </=50 x109/L, moderate 51-99 x109/L, mild >/=100 x109/L). Our secondary outcome was the identification of platelet transfusion triggers according to degree of anticoagulation intensity.
Results
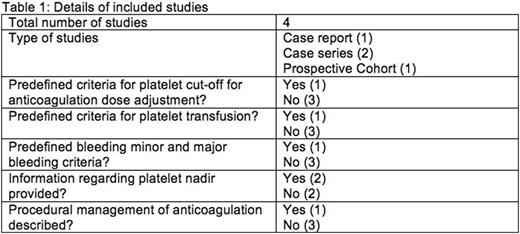
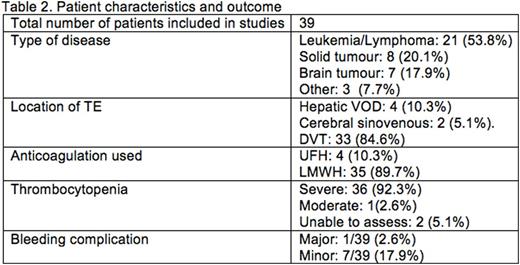
Our search yielded 244 articles, of which 13 were screened at a full text level. Four manuscripts were selected for inclusion, as follows: case report (n=1), case series (n=2), and prospective cohort study (n=1). Details of included studies are described inTable 1 and patient information in table 2. The studies included 39 patients with malignancies, of which the most common were acute lymphoblastic leukaemia (n=13), CNS malignancies (n=6), non-hodgkinlymphoma(n=4). All patients had venous TE. Type and regimen of ACT, and author definitions of thrombocytopenia and bleeding were variable. Thirty-five patients received low-molecular weight heparin (LMWH), of whom 33 received therapeutic dose, and no dose was recorded for two. Three patients received unfractionated heparin (UFH) with antithrombin(AT) supplementation; however, UFH wasstopped in periods of low platelet counts, and one received a reduced dose UFH. According to our definition, 36/39 (92.3%) experienced severe thrombocytopenia, 1 (2.6%) developed moderate thrombocytopenia, platelet count was not stated in 2(5.1%). Using the ISTH bleeding criteria, one patient (2.6%) developed major bleeding, and seven (17.9%) developed minor bleeding (n=2 intestinal bleeding, n=5 injection site/port bleeding). Only one study reported our secondary outcome, which used a platelet transfusion trigger of <40x109/L if receiving therapeutic LMWH and <20x109/L if receiving prophylactic LMWH. Given the heterogeneity, we were unable to statistically aggregate any data.
Discussion
The results of this systematic review highlight the paucity of high-quality data to assess the use and safety of ACT for TE in paediatric cancer patients during periods of thrombocytopenia. We identified only observational studies- one prospective and three retrospective in design. These studies included very few patients (total n=39), and had variable patients, disease and treatment-related factors. Evidence-based recommendations cannot be generated to guide an optimal ACT management in such situations. Further research using standardized definitions is needed to identify optimal strategies for treating thrombotic events during periods of thrombocytopenia. Such research will help on the development of evidence-based clinical practice guidelines to improve patient care.
Brandão:Boehringer Ingelheim: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal