Abstract
BACKGROUND: The link between aging, cancer and atrial fibrillation is well known and the appropriate anticoagulation management of non-valvular atrial fibrillation (NVAF) in patients with active cancer is of growing clinical concern. Rivaroxaban has been broadly used for the primary prevention of stroke and systemic embolism in the general population of patients with NVAF. However, individuals with a serious concomitant illness associated with a life expectancy of less than 2 years were excluded from pivotal trials including the ROCKET-AF study, limiting the generalizability of results to patients with active cancer. There is little published evidence on the safety and efficacy of rivaroxaban for NVAF in this specific subgroup.
OBJECTIVES: The aim of this study was to assess the safety and efficacy of rivaroxaban in patients with active cancer and NVAF.
METHODS: The use of rivaroxaban in cancer patients at Memorial Sloan Kettering Cancer Center (MSKCC) was monitored in the setting of a Quality Assessment Initiative. Patients with active cancer and NVAF treated with rivaroxaban from 1/1/2014 through 3/31/2016 are included in this analysis. Endpoints of interest were defined a priori and include stroke, systemic embolism, major bleeding and clinically-relevant non-major bleeding leading to discontinuation of rivaroxaban for at least 7 days (CRNMB). Clinical events were assessed through text searches of medical records. The analysis was performed respecting different times on previous anticoagulation, considering the first 90 days as the acute phase of treatment and contrasting it with the subsequent chronic phase.
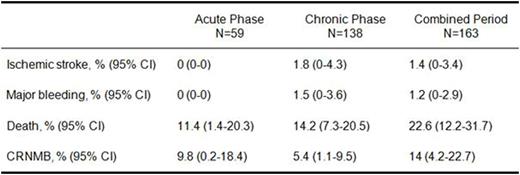
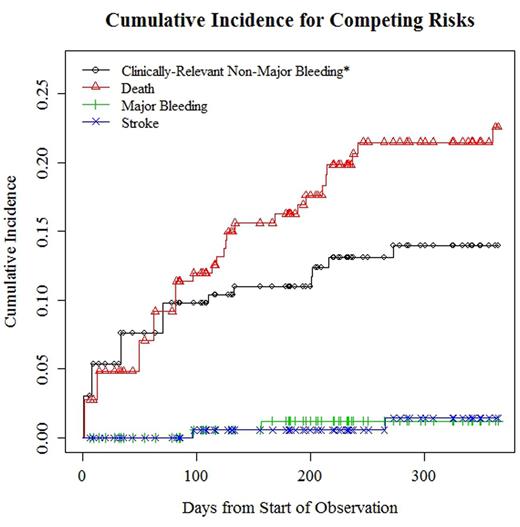
RESULTS: A total of 163 evaluable patients were included in the analysis, with a median age of 72.0 years (interquartile range=67.0-77.5 years) and 56% of these individuals being men. The majority had a solid tumor (85%), with stage IV disease reported in 50% of cases. The mean CHA2DS2-Vasc score was 3.2 (standard deviation=1.5) and 64% of patients were already in the chronic phase of anticoagulation. Results for the acute, chronic and combined phases of anticoagulation are presented in the Table and plotted in the Figure. The estimated cumulative incidence of ischemic stroke, major bleeding, and CRNMB at 1 year were 1.4% (95% CI=0-3.4%), 1.2% (95% CI=0-2.9%) and 14.0% (95% CI=4.2-22.7%) respectively, after adjusting for competing risks. Interestingly, the cumulative incidence of major bleeding in our cohort is lower than the value reported for the ORBIT-AF registry of cancer patients on dabigatran or a vitamin K antagonist for NVAF, in which the estimated rate of this complication was 5.1/100 patient-years. Lastly, the 1-year cumulative incidence of mortality was 22.6% (95% CI=12.2-31.7%). This high risk of death was present throughout the observation period and reflects the cancer population. One patient died after developing an acute ischemic cerebrovascular insult and a myocardial infarction. There were no deaths related to bleeding and no recorded systemic embolism episodes.
CONCLUSIONS: The safety and efficacy profile of rivaroxaban treatment for NVAF in patients with active cancer seems comparable to what was observed for the general population in the ROCKET-AF study, but ideally a prospective study would be required to confirm these findings.
Cumulative Incidence of Competing risks for Patients in the Acute, Chronic and Combined Phases of Anticoagulation*
*Cumulative incidence estimates for the chronic phase are conditional to reaching day 90 of anticoagulation without sustaining an event. The chronic phase was defined as lasting 275 days and the combined period encompasses 365 days.
CRNMB: Clinically-relevant non-major bleeding leading to discontinuation of rivaroxaban for at least 7 days.
*Clinically-relevant non-major bleeding leading to discontinuation of rivaroxabanfor at least7 days.
Cumulative Incidence of Competing risks for Patients in the Acute, Chronic and Combined Phases of Anticoagulation*
*Cumulative incidence estimates for the chronic phase are conditional to reaching day 90 of anticoagulation without sustaining an event. The chronic phase was defined as lasting 275 days and the combined period encompasses 365 days.
CRNMB: Clinically-relevant non-major bleeding leading to discontinuation of rivaroxaban for at least 7 days.
*Clinically-relevant non-major bleeding leading to discontinuation of rivaroxabanfor at least7 days.
Yu:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Soff:Janssen Scientific Affairs, LLC: Consultancy, Research Funding. Mantha:Janssen Scientific Affairs, LLC: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal