Abstract
INTRODUCTION
The unpredictable clinical response of patients to bypassing therapy and the lack of a proper laboratory tools to measure clot formation and stability renders prophylaxis and surgery on these patients a huge challenge. These patients are at a risk for bleeding or thromboembolic complications.
AIMS
In this study we introduce a novel plasma based microfluidic assay that can qualitatively and quantitatively measure fibrin deposition, thrombin and plasmin generation, and fibrinolysis under flow.
We then examined the dynamics of thrombus formation in patients with hemophilia and their response to replacement and bypass therapies under flow conditions.
METHODS
Coagulation in the plasma based assay was initiated by spherical 1µm lipidated- Tissue Factor biomimetic silica beads which were patterned into 200µm circles on a substrate surface. Plasma samples were obtained from Hemophilia patients and inhibitor patients, before and after replacement or bypassing treatment and perfused over the tissue factor rich surfaces at a sheer rate of 100 s-1 Fibrinolysis was initiated with the addition of tPAto the plasma samples before perfusion.
RESULTS
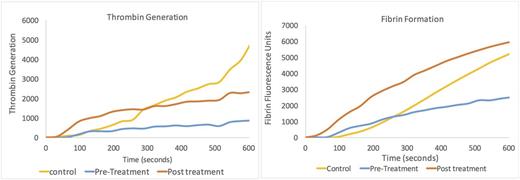
The microfluidic assay was sensitive enough to measure the activation of coagulation triggered by the bypassing agents. Fibrin generation and thrombin generation were measurable both qualitatively and quantitatively using three metrics; lag time, rate of production and maximum quantity produced. Individuals on replacement therapy showed normalized fibrin formation with a 69% increase in fibrin formation, a decrease in lag time and an overall increase in maximum fibrin and thrombin production (See attached Figure).
The microfluidic assay was also able to show an increase in overall fibrin generation in certain Individuals that were given more bypassing treatment than needed. Compared to healthy controls the rate of fibrin generation and maximum fibrin was greater, thereby identifying a risk for prothrombotic state. (See attached Figure). Finally, using the microfluidic assay we were able to observe both clot formation and lysis and asses the the stability of the fibrin clot produced when these inhibitor patients were on and off treatments, which reflects a more complete picture of the coagulation process.
CONCLUSION
We are able to show that individuals, treated by replacement therapies showed normalized clot formation. Individuals with hemophilia treated by bypassing therapies also showed normalized clot formation. Sometimes however, the fibrin production is more than a normal control, which could lead to a risk of prothrombosis. By using microfluidic assay, the treatment can be given in doses and fibrin production observed, to decrease the overall fibrin formation from a hypocoagulable to a hemostatic state, avoiding hypercoagulability.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal