Abstract
Von Willebrand factor (VWF) levels vary over time and increase throughout life in both healthy individuals and patients with von Willebrand disease (VWD). Especially in type 1 VWD patients, this increase may result in normalization of VWF levels. It is not yet known if normalization of VWF levels ameliorates bleeding symptoms in VWD patients. We have recently shown that elderly type 1 VWD patients had similar bleeding tendency as younger adults.1 However, many elderly patients in this study had relatively low VWF levels and many younger adults had relatively high VWF levels.1 The aim of the current study was to investigate the association between normalization of VWF levels and the bleeding phenotype in type 1 VWD patients.
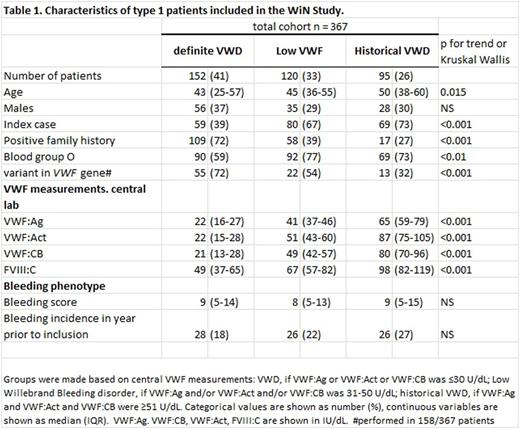
We included patients from the nationwide cross-sectional "Willebrand in the Netherlands" Study, with lowest historical VWF antigen (VWF:Ag) and/or VWF activity (VWF:Act) ≤30 U/dL. At inclusion, blood was sampled for central measurement of VWF:Ag and VWF:Act and VWF to collagen binding (VWF:CB). Central measurements were available in 367 type 1 VWD patients.
Based on these central measurements, patients were divided into three groups: definite VWD (central VWF:Ag and/or VWF:Act and/or VWF:CB ≤30 U/dL, n=152); low VWF (central VWF:Ag and/or VWF:Act and/or VWF:CB 31-50 U/dL , n=120) and historical VWD with presently normalized levels (central VWF:Ag and VWF:Act and VWF:CB ≥51 U/dL, n=95).
Age differed between groups: median age was 43 years in definite VWD patients, 45 years in low VWF and 50 years in historical VWD patients (p<0.01). No difference in sex distribution was found, see table 1. Of definite VWD patients, 59% had blood group O compared with 77% of low VWF and 73% historical VWD patients (p<0.01). A variant in the VWF gene was most common in definite VWD patients: 55/76 (72%) patients in whom mutation analysis was performed had a variant compared with 22/41 (54%) low VWF and 13/41 (32%) historical VWD patients (p<0.001). Of definite VWD patients, 72% had a positive family history, compared with 39% of low VWF and 27% of historical VWD patients (p<0.001). In contrast, 69% of historical VWD patients were index cases, compared with 67% of "low VWF" and 39% of definite VWD patients (p<0.001).
Median Tosetto Bleeding Score (BS) did not differ between the three groups as it was respectively 9, 8 and 9 in definite VWD patients , low VWF patients and historical VWD patients (p=NS), see table 1. The incidence of bleeding episodes requiring treatment with desmopressin or clotting factor concentrate in the year prior to inclusion also did not differ between groups as it was 18% in definite VWD, 22% in "low VWF" and 27% in historical VWD patients (p=NS). In many countries VWF levels 60 IU/dL are used as the cut-off value for abnormal VWF levels. Using this value as cut-off between low VWF and historical VWD, 151 (42%) patients had low VWF and 64 (17%) patients had historical VWD. Using this cut-off value had no major effect on bleeding phenotype or patient characteristics (data not shown).
In conclusion, patients with higher VWF levels at the time of study were older and less often had a variant in the VWF gene. More historical VWD patients were index cases, underlining their negative family history. In contrast, blood group O was more common in patients with normalized VWF levels, suggesting that factors outside the VWF gene have a more important effect on VWF levels in this group. Importantly, the bleeding score was similar in all groups. The bleeding score is a reflection of bleeding symptoms that have occurred throughout one's life and may therefore not detect changes in bleeding phenotype over time. Importantly, the bleeding incidence requiring treatment in the year prior to inclusion was also similar in all groups, regardless of VWF levels. Our study suggests that normalization of VWF levels is not associated with the bleeding phenotype in type 1 VWD patients. However, this study is limited by its retrospective design and prospective studies are required to assess the bleeding phenotype and bleeding rate in more detail and to identify patients at increased or decreased risk of bleeding.
1Sanders YV, Giezenaar MA, Laros-van Gorkom BA, et al. von Willebrand disease and aging: an evolving phenotype. J Thromb Haemost. 2014;12(7):1066-1075.
Boender:CSL Behring: Research Funding. Eikenboom:CSL Behring: Research Funding. Fijnvandraat:CSL Behring: Research Funding; Bayer: Research Funding. Meijer:Bayer: Honoraria, Research Funding; Baxter: Research Funding; Pfizer: Research Funding; Sanquin: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria; Bristol-Myers Squibb: Honoraria. Mauser-Bunschoten:Baxter: Research Funding; Bayer: Research Funding; CSL Behring: Research Funding; Novo Nordisk: Research Funding; Griffols: Research Funding; Sanquin: Research Funding. Cnossen:Novo Nordisk: Research Funding; CSL Behring: Other: Travel Funding, Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Baxalta: Research Funding; Bayer: Research Funding. Laros-van Gorkom:CSL Behring: Research Funding. van der Bom:CSL Behring: Research Funding; Novo Nordisk: Research Funding; Pfizer: Research Funding; Bayer: Research Funding; Baxalta: Research Funding. Leebeek:UniQure: Consultancy; Netherlands Hemophilia Foundation: Research Funding; Baxter: Research Funding; CSL Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal