Abstract
Background
Prophylaxis is increasingly the standard of care for adults with severe hemophilia A (SHA), however the optimal dose and treatment regimen when initiating tertiary prophylaxis is unknown. The relationship between factor level and risk of bleeding is not established, particularly in patients with joint disease. A post hoc analysis was conducted on a cohort of adult SHA patients who switched to prophylaxis from on demand therapy.
Methods
The cohort included 63 subjects with measured factor VIII (FVIII) levels of <1 IU/dL, aged 7 to 59 years, receiving on-demand treatment and with a minimum of eight joint hemorrhages in the 12 months prior to enrollment. After 6 months observation of on demand treatment and a full pharmacokinetic (PK) analysis, subjects were randomized to 12 months of either standard prophylaxis (20-40 IU kg−1 every 2nd day) or PK-tailored prophylaxis (20-80 IU kg−1 every 3rd day) targeting FVIII trough levels ≥1 IU/dL.
Subjects had a median age of 28 years, and median weight of 71Kg. At study start, 95.2% of subjects had ≥1 target joints (median 3). During on demand treatment before randomization, the median number of spontaneous-joint bleeds was 29.9/year (range: 0 to 91.3). During prophylaxis, the median number of spontaneous-joint bleeds was 0/year (range: 0 to 10.2) in the combined prophylaxis groups.
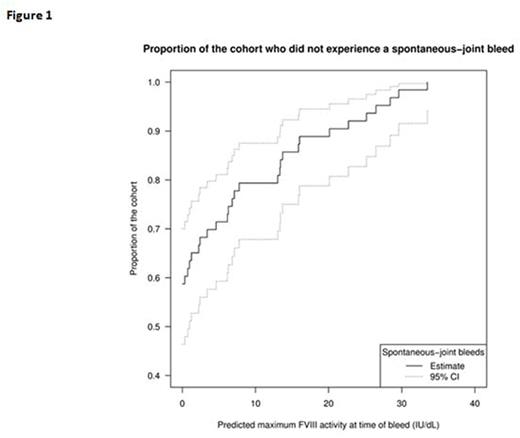
A PK model was fitted to the FVIII activity levels collected during the PK analysis for each subject independently. These individual models were used to predict the FVIII activity level at the time of bleed on the assumption of linear pharmacokinetics and no inter-occasion variability. For each subject the highest FVIII activity level associated with a spontaneous-joint bleeding event was considered as the minimally effective level to prevent spontaneous-joint bleeds. A Kaplan-Meier estimate for the proportion of subjects with no spontaneous-joint bleeds above FVIII activity level was carried out. For subjects who had no spontaneous-joint bleeding events an assumption was made that they would have bled only at 0 IU/dL, even in situations where the trough levels may have been higher.
Results
The Kaplan-Meier curve for proportion of the cohort without spontaneous-joint bleeds as function of predicted FVIII level is presented in Figure 1. The corresponding estimates are presented in Table 1. Based on this analysis in this cohort, the majority of patients (63%) would not be expected to have spontaneous-joint bleeds while maintaining a trough level of 1 IU/dL with more marginal improvements in response to higher FVIII levels. These results indicate that, in subjects with multiple target joints starting tertiary prophylaxis, most experience no spontaneous-joint bleeds for a predicted FVIII level ≥1 IU/dL, however, bleeds occurred at much higher predicted FVIII levels in some subjects. This is within the range of FVIII levels associated with hemophilia (0-40%) (White GC, et al. Thromb Haemost. 2001 Mar; 85(3):560).
Conclusion
The results of the model and the present post-hoc analysis support current trends to personalize prophylaxis based on individual variations in bleeding phenotypes, despite similar FVIII levels. To prevent all spontaneous-joint bleeds, some patients may require higher levels, findings which are congruent with those published on the natural history of joint bleeding in mild, moderate, and severe hemophilia patients (Den Uijl IE et al. Haemophilia. 2011;17(6):849-853).
Fischer:Wyeth/Pfizer: Research Funding; Biogen: Consultancy; NovoNordisk: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Biotest Octapharma: Speakers Bureau; CSL Behring: Speakers Bureau; Baxter: Consultancy, Research Funding, Speakers Bureau; Freeline: Consultancy; Bayer: Consultancy, Research Funding, Speakers Bureau. Chowdary:Sobi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Biogen Idec: Consultancy, Honoraria; Baxalta: Consultancy, Honoraria; Bayer: Honoraria. Collins:NovoNordisk: Consultancy; Sobi: Consultancy; CSL Behring: Consultancy, Research Funding; Shire: Consultancy, Speakers Bureau. Cotterill:Shire: Employment. Pipe:UniQure: Consultancy; Pfizer: Consultancy; Genentech/Roche: Consultancy; Biogen: Consultancy; CSL Behring: Consultancy; Novo Nordisk: Consultancy; Shire: Consultancy, Other: Grant funding. Wolfsegger:Shire: Employment, Equity Ownership. Engl:Shire, formerly Baxalta and Baxter: Employment, Equity Ownership. Goldstine:Shire: Employment. Valentino:Shire: Employment. Spotts:Shire: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal