Abstract
INTRODUCTION
Congenital FXIII A-subunit deficiency is a rare, autosomal recessive coagulation disorder with a high risk of life-threatening bleeding complications such as intracranial hemorrhages (Muszbek & Katona. Semin Thromb Hemost 2016;42:429-439). The true prevalence is estimated to be ~1 in 1 to 2 million people worldwide (Anwar R, et al. Pediatrics 2002;109:E32), however, there are only ~1300 diagnosed patients in the world (World Federation of Haemophilia. Report on the Annual Global Survey 2014), suggesting significant under-diagnosis.
When severe congenital FXIII deficiency is confirmed, prophylactic replacement therapy is strongly recommended as without prophylaxis, FXIII deficiency places patients at high risk of spontaneous fatal or devastating intracranial bleeding, and because FXIII prophylaxis is reasonably easy and infrequent (once monthly) due to the long half-life of FXIII concentrates. Recombinant FXIII (rFXIII) is a new treatment possibility (Dorey E. Nat Biotechnol 2014;32:210), and here we report long-term safety and efficacy results from mentor™2, the largest clinical trial performed to date in patients with congenital FXIII A-subunit deficiency treated with rFXIII.
METHODS
The mentor™2 trial was a multinational, open-label, single-arm, multiple-dosing safety extension to the pivotal mentor™1 trial (Inbal A, et al. Blood 2012;119:5111-5117). The trial period was from September 2009 to October 2015. Eligible patients were aged ≥6 years, weighed ≥20 kg, and were diagnosed with congenital FXIII A-subunit deficiency. The Berichrom® FXIII activity assay was used to measure FXIII activity. The trial aimed to assess the long-term safety of replacement therapy with rFXIII (35 IU/kg once monthly) when used for the prevention of bleeding episodes and the treatment of breakthrough bleeds.
RESULTS
Sixty patients (64% male) were enrolled and exposed to rFXIII; 34 from the mentor™1 trial, and 26 newly recruited patients. Median age of patients at enrollment was 26.0 years (range 7.0-77.0) and 16 (27%) were <18 years old. Patients were exposed to a total of 2410 rFXIII doses and were followed for a total of 186.5 patient-years.
Safety: a total of 920 treatment-emergent adverse events (AEs) were reported in 56 of the 60 (93.3%) patients: headache and nasopharyngitis occurred most commonly. No anti-rFXIII neutralizing or non-neutralizing antibodies were detected. In the mentor™1 trial 4 patients developed non-neutralizing antibodies, this was not seen in the current mentor™2 trial. There were no thromboembolic events, fatal AEs, or AEs leading to withdrawal, and no anaphylactic or allergic reactions to rFXIII. Serious AEs were few (19 events in 12 patients) and were evaluated as unlikely to be related to rFXIII. There were 7 AEs in 7 patients that were evaluated as possibly/probably related to the trial product (incorrect dose, overdose, arthralgia, leukopenia, limb injury, alanine aminotransferase increase, and blood alkaline phosphatase increase); all patients recovered. The case of overdose was 2.3 times the planned dose; the patient did not experience any adverse consequences.
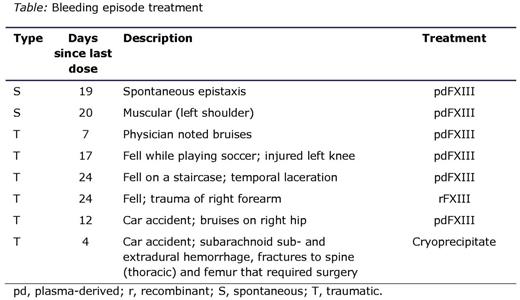
Efficacy: in all, 8 FXIII treatment-requiring bleeds occurred in 7 patients: 6 were trauma induced and 2 were spontaneous (Table). Three of the 8 bleeds occurred within the first 14 days post rFXIII infusion when FXIII levels would have been predicted to be >30% in all cases, while 5 bleeds occurred between days 17 and 24 post rFXIII infusion. No internal organ bleeds or severe gastrointestinal bleeds occurred. One trauma-induced muscular bleed was treated with rFXIII with an excellent response. The annual mean bleeding rate was 0.043 bleeds/patient/year, implying 1 bleed per patient, per ~23 years (0.032 for traumatic bleeds and 0.011 for spontaneous bleeds). The geometric mean FXIII trough level was 0.17 IU/mL (coefficient of variation of 0.37).
CONCLUSION
This is the largest dataset on congenital FXIII A-subunit deficiency, both in terms of number of patients and number of patient-years. Over the 6-year trial duration, no safety issues were identified and very few FXIII treatment-requiring bleeding episodes occurred, mainly trauma-related. These data all point to the safety and efficacy of a prophylactic regimen of 35 IU/kg of rFXIII given every 4 weeks to manage congenital deficiency of FXIII A subunit.
Carcao:Biogen/Idec/Sobi: Honoraria, Research Funding; Baxalta: Honoraria; Bayer: Honoraria; CSL Behring: Honoraria, Research Funding; Novo Nordisk: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Octapharma: Honoraria. Altisent:Octapharma: Consultancy; CSL Behring: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Grifols: Consultancy; Novo Nordisk: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Baxalta: Consultancy, Research Funding. Castaman:Kedrion: Membership on an entity's Board of Directors or advisory committees; Baxalta-Shire: Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Sobi: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Fukutake:simic: Research Funding; Sekisui Medical: Consultancy, Honoraria, Speakers Bureau; Roche Diagnostics: Honoraria, Speakers Bureau; Biogen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbott: Honoraria, Speakers Bureau; Kaketsuken: Honoraria; Japan Blood Products Organization: Honoraria, Research Funding; Torii: Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; LSI Medience: Consultancy; SRL Inc: Consultancy; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; CSL Behring: Honoraria; EPS: Research Funding; Siemens: Speakers Bureau. Kerlin:Bayer Healthcare US: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring Foundation: Research Funding. Kessler:Pfizer: Consultancy; Octapharma: Consultancy, Research Funding; Novo Nordisk: Consultancy, Research Funding; Grifols: Consultancy; Genentech: Consultancy, Research Funding; Biogen: Consultancy; Bayer: Consultancy, Research Funding; LFB: Other: Member of DSMB; Baxalta: Consultancy, Research Funding. Lassila:Aplagon Oy, Finland: Consultancy, Patents & Royalties. Rosholm:Novo Nordisk: Employment, Equity Ownership. Garly:Novo Nordisk: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal