Abstract
Introduction: Fetal-NeonatalAlloimmune Thrombocytopenia (FNAIT) is the result of an incompatibility between maternal and fetal human platelet alloantigens (HPA). Affecting 1 in 1000 births, it is one of the most common causes of thrombocytopenia in the neonate. Prospective studies reveal that only 5-10% of pregnancies with HPA-1a incompatibilities result in maternalalloimmunization. The association of HPA-1a incompatibility andalloimmunization is largely based on an immune response gene: the Human Leukocyte Antigen (HLA) class II DRB3*0101 haplotype. Clinical studies have shown that >90% of women that produce HPA-1a antibodies express HLA-DRB3*0101. The HLA-DRB3*0101 haplotype is found on the HLA-DR3 locus of HLA-A1-B8-DR3 - amultigene haplotype which has been implicated in the development of a number of autoimmune diseases. The presence of HLA-A1-B8-DR3 has been shown to impact susceptibility to, the age of onset, and severity of autoimmune diseases. In this study we seek to investigate whether the HPA-1aalloimmunization that results in FNAIT, which is linked to the autoimmune haplotype, is also associated with an increased incidence of maternal autoimmune diseases following pregnancy.
Methods: A retrospective review was conducted to identify patients with HPA-1a cases of FNAIT associated pregnancies. Patients with serologically confirmed anti-HPA-1a FNAIT completed a general health status survey developed by our laboratory in 2013 (n = 51; mean age = 30.6 years). In addition to questions about their health, patients described the course of their pregnancy, complications, and any health problems that followed their pregnancy. A follow-up survey was conducted in 2015 (n = 49, mean age = 33.4 years) with additional questions focusing on the development of autoimmune diseases. The incidence of autoimmune disease in our sample population was compared with that in the general population controlled for age and gender (Centers for Disease Control; Table 1).
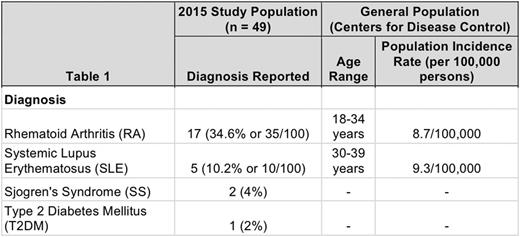
Results: Analysis revealed that of the 49 patients contacted in 2015, 25 patients (51%) had a confirmed diagnosis of an autoimmune disease. Patients reported Rheumatoid Arthritis (RA) (34.6%), Systemic LupusErythematosus (SLE)(10.2%),Sjogren's Syndrome (SS) (4%), and Type 2 Diabetes Mellitus (T2DM) (2%). When compared with the general population, controlled for age and gender, there was an increased incidence of both RA and SLE in the study population.
Conclusions: Our results reveal that patients with HPA-1aalloimmunization have indeed developed autoimmune diseases or symptoms that may suggest an autoimmune disease since initial contact in 2013. Specifically, of the 49 patients that were contacted in 2015 and completed the autoimmune disease questionnaire, 25 had confirmed diagnoses of an autoimmune disease. Given the role of the HLA-DR3*0101 haplotype in HLA-1aalloimmunization and that of HLA-A1-B8-DR3 in the development of autoimmune disease, this data suggests that women who do not encode for HPA-1a (i.e., those that express HPA-1b1b) and develop HPA-1aalloimmunization may be susceptible to the development of autoimmune disease(s) later in life. These findings will be compared with women who do not encode for HPA-1a (i.e., those that express HPA-1b1b) but do not make anti-HPA-1a antibodies.Future studies can be aimed at investigating not only the frequency with which autoimmune diseases occur followingalloimmunization but also if they can be prevented.
Bussel:Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; Symphogen: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Research Funding; BiologicTx: Research Funding; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate: Patents & Royalties; Prophylix Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Momenta Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees; Sysmex: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal