Abstract
Introduction:
Heparin-Induced thrombocytopenia (HIT) is a potentially life-threatening, immune complex mediated adverse reaction of heparin therapy, which may be suspected in patients who develop thrombocytopenia and/or thrombosis during, or following exposure to, unfractionated or low molecular weight heparin. Laboratory demonstration of heparin-platelet factor 4 (HPF4) antibodies supports more timely cessation of heparin therapy and initiation of alternative anticoagulation, and is an essential component of the diagnosis of HIT. The serotonin release assay (SRA) is considered the gold standard, but is technically difficult, requires normal donor platelets and radioactive isotopes. As a result, the SRA is not widely available in routine clinical laboratories. Enzyme immunoassays (EIA) are commonly used to support an HIT diagnosis; however, currently available EIAs typically require sample batching, which usually delays HIT diagnosis, and may be unavailable in laboratories with low test volumes. We evaluated the HemosIL HIT-Ab(PF4-H) (Instrumentation Laboratory, Bedford, MA) assay, a rapid, on-demand, automated method that can be performed 24/7 and provides results more quickly than existing tests.
Methods:
The assay cut-off determination was performed by a method comparison with SRA on HIT-suspected plasma samples (n=63; 31 SRA-positive and 32 SRA-negative). The reference interval was calculated using citrated plasma samples from normal donors (n=131) and HIT-suspected patients that were negative by EIA (n=122). A multicenter study of HemosIL HIT-Ab(PF4-H) assay on ACL TOP Family systems was conducted between March 2013 and June 2015. Method comparisons were conducted between HemosIL HIT-Ab(PF4-H), Asserachrom HPIA assay (Diagnostica Stago, Asnieres, France) (n=632), and SRA (n=537). HemosIL HIT-Ab(PF4-H) was also compared with clinical probability using the 2013 American Society of Hematology (ASH) guideline, a diagnostic algorithm that classifies samples as (HIT Likely) or (HIT Unlikely) based on the 4T score, EIA, and SRA results.
Results:
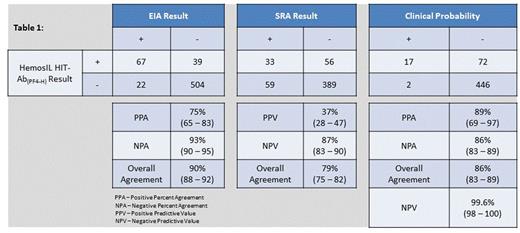
The optimal cut-off determined by Receiver Operator Curve (ROC) analysis was 1.0 U/mL (95.2% agreement, 95% confidence interval (CI) 86.7-99.0). The 95% reference interval was 0.0-0.7 and 0.0-0.9 U/mL for healthy donors and HIT negative suspected patients, respectively. Thus, based on these results, a value of equal or greater than 1.0 U/mL was considered positive for HIT antibodies. The overall agreement of HemosIL HIT-Ab(PF4-H) and EIA and SRA results were 90% (95% CI 88-92%), and 79% (95% CI 75-82%), respectively (Table 1). The overall agreement of the clinical probability compared to HemosIL HIT-Ab(PF4-H) results was 86% (95% CI 83-89%) with a Negative Predictive Value (NPV) of 99.6% (95% CI 98-100%) (Table 1).
Discussion:
Timing is critical in HIT diagnosis and affects clinical decisions in HIT-suspected patients. The advantage of an on-demand, automated assay is that test results can be provided in a more timely fashion, even in a routine clinical laboratory. Our data show that the HemosIL HIT-Ab(PF4-H) results in HIT-suspected patients are comparable to EIA and SRA. In addition, substantial correlation is noted between HemosIL HIT-Ab(PF4-H) results and the clinical probability of HIT with a NPV approaching 100%, which demonstrates the clinical utility of this assay in excluding a diagnosis of HIT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal