Abstract
Introduction: Atypical hemolytic uremic syndrome (aHUS) is a complement-mediated disorder classically defined as microangiopathic hemolytic anemia, thrombocytopenia, and renal dysfunction. Defects in complement regulation may be inherited or acquired, resulting in chronic activation of complement - the pathophysiologic basis of aHUS. aHUS is classified as a rare disorder; however available epidemiologic data is based on pediatric or post-transplant populations. The incidence and prevalence of aHUS remains unknown in the general adult population. Clinical recognition of aHUS may be obscured or delayed due to the absence of a diagnostic test and unfamiliarity with the diverse presentations of these patients. Complement-amplifying conditions (CACs) often unmask aHUS in patients with defects in complement regulation (Kavanagh et al. Br Med Bull 2006). The co-existence of aHUS and CACs frequently presents additional diagnostic challenges. Data demonstrates that earlier recognition and treatment of aHUS with eculizumab, a humanized monoclonal antibody that binds to C5 inhibiting terminal complement activation, leads to better overall outcome for patients. (Legendre et al. NEJM 2013)
Methods: We performed a retrospective chart review of all patients treated with eculizumab for clinically diagnosed aHUS at Medstar Georgetown University Hospital (MGUH) between December 2011 and January 2016.
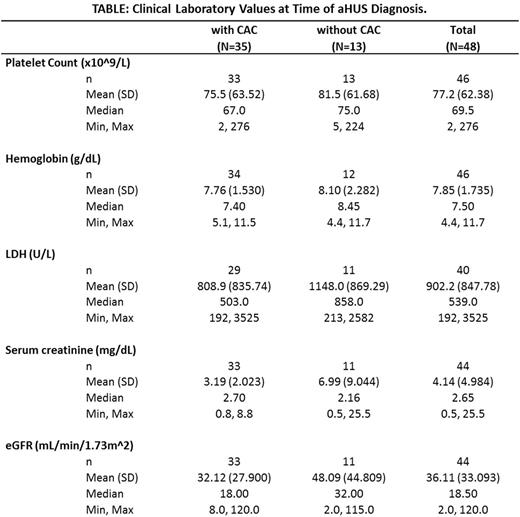
Results: 48 patients received at least one dose of eculizumab for the clinical diagnosis of aHUS at our center between 2011 and 2015. Median age at diagnosis was 46 years (range: 19 to 71 years) with a slight female-to-male predominance (27 to 22, respectively). Seventy three percent (35/48) of patients had identifiable complement-amplifying conditions (CACs) at diagnosis. Identified CAC categories include 11% (5/48) autoimmune/mixed connective tissue disease, 6% (3/48) infections, 4% (2/48) HIV, 4% (2/48) chemotherapy exposure, 40% (19/48) non-renal solid-organ transplant, 10% (5/48) renal transplant, 4% (2/48) surgery, and 8% (4/48) inflammatory bowel disease. Ninety four percent (45/48) of patients had evidence of 1 or more extra-renal manifestations of aHUS at the time of diagnosis. Six percent (3/48) of patients presented with renal involvement only, compared with 23% (11/48) who had 1 extra renal manifestation, 18% (9/48) with 2 extra renal manifestations, 35% (17/48) with 3 extra renal manifestations, and 17% (8/48) with 4 or more extra renal manifestations. Extra renal systems involved include cardiovascular (67%), pulmonary (65%), gastrointestinal (58%), and CNS (42%). Patients with CACs presented with a similar distribution of extra-renal manifestations compared with patients who had no identified CACs. No significant difference was observed in presenting hematologic and renal labs, but we observed a trend towards more severe renal involvement at presentation in those without CACs (see Table). Regardless of CAC status, similar proportions of patients were on dialysis prior to or at the time of eculizumab initiation. Time to recognition and initiation of eculizumab in hospitalized patients with CACs took an average of 37 days (median 15 days) compared with an average of 17 days (median 7 days) in those without CACs.
Conclusions: aHUS was diagnosed in patients in all age-groups and affected both sexes with similar frequency. We confirm other reports of a significant association between CAC and aHUS with 73% of our patients presenting with a CAC at the time of diagnosis of aHUS. While the majority of patients with and without CACs (91% and 100%, respectively) presented with extra-renal manifestations, those with CACs experienced less-severe renal involvement compared to those with no CACs. Patients with comorbid CACs were diagnosed and initiated on eculizumab later than those without CACs. Our experience emphasizes the importance of recognizing early non-renal systemic manifestations of thrombotic microangiopathy to make the diagnosis of aHUS, especially in patients with comorbid CACs.
Wang:Alexion Pharmaceuticals: Employment. Broome:Alexion Pharmaceuricals: Honoraria; True North Therapeutics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal