Abstract
Introduction: Disorders of coagulation are common in sepsis, with disseminated intravascular coagulation (DIC) occurring in approximately 35 % of severe cases, contributing to microvascular dysfunction and death. Intensive platelet activation in sepsis facilitates platelet aggregation, leading to the formation of microthrombi and platelet depletion. This results in the development of DIC and sepsis-associated thrombocytopenia. Therefore, platelets must be cleared locally and quickly in the early phase of activation. Previous studies mainly focused on the clearance of activated cold-stored and aging platelets as well as platelets in immune-mediated thrombocytopenia. However, platelet activation and their clearance in sepsis are poorly understood. Platelets can form aggregates with leukocytes resulting in leukocyte death, the release of extracellular traps (ETs), increased endothelial permeability, and aggravated thrombosis. This study explored an alternate pathway for platelet disposal mediated by endothelial cells (ECs) through phosphatidylserine (PS) and examined the effect of platelet clearance on procoagulant activity (PCA) in sepsis.
Methods: The subjects were septic patients (n=48) and healthy controls (n=48). Platelet engulfment by ECs was observed by electron microscopy, immunofluorescence, or immunochemistry both in vitro and in animal models. The PCA of platelets was measured by clotting time, purified coagulation complex assays, and fibrin formation.
Results: Platelets in septic patients demonstrated increased levels of surface activation markers and apoptotic vesicle formation, and also formed aggregates with leukocytes. Activated platelets adhered to and were ultimately digested by ECs in vivo and in vitro. Blocking PS on platelets or integrin on ECs attenuated platelet clearance, resulting in increased platelet count in a mouse model of sepsis (p<0.05). Furthermore, platelet removal by ECs resulted in a corresponding decrease in platelet-leukocyte complex formation and markedly reduced generation of factor Xa and thrombin on platelets (p<0.01). Pretreatment with lactadherin increased phagocytosis of platelets by approximately 2-fold, diminished PCA by 70%, prolonged coagulation time, and attenuated fibrin formation by 50%. A large decline in PS exposure on platelets, associated platelet PCA, and PLA formation is seen in patients in remission, which could be attributed to the elimination of abnormal platelets.
Conclusions: Our results suggest that PS-mediated clearance of activated platelets by the endothelium results in an anti-inflammatory, anticoagulant, and antithrombotic effect that contributes to maintaining platelet homeostasis during acute inflammation. Antiplatelet treatment has been suggested as a novel strategy in sepsis, and we speculate that promoting efficient removal of activated and apoptotic platelets could further improve patient outcomes. Therefore, clearance of activated platelets earlier in the disease process could hasten recovery of homeostasis in circulation by eliminating catalytic platforms for the coagulation pathway, protecting blood cells from excessive activation, and restoring their normal function. Endothelium, at least in part, contributes to platelet disposal and may further improve the hypercoagulable status in inflammation. It is noteworthy that PS-mediated and lactadherin-strengthened platelet engulfment may modify coagulopathy, and thus provide a new modality for treatment of septic clotting disorders.
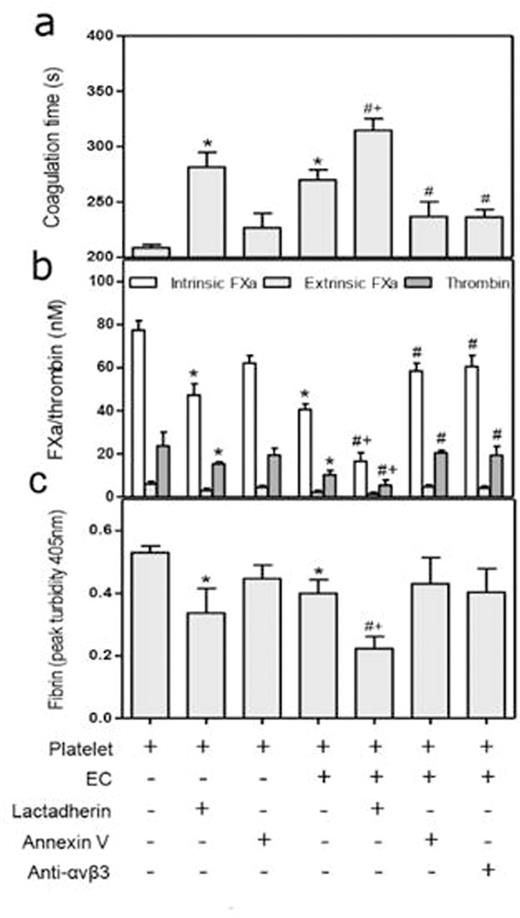
Effect of lactadherin-mediated phagocytosis on procoagulant activity and fibrin formation.
Effect of lactadherin-mediated phagocytosis on procoagulant activity and fibrin formation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal