Abstract
Background: ALXN1210 is a humanized monoclonal antibody designed for rapid, complete, sustained inhibition of C5 with less frequent dosing. A previous study in healthy volunteers demonstrated immediate, complete, and sustained C5 inhibition. The terminal half-life of ALXN1210 is approximately 3-4x longer than eculizumab, thus providing continuous suppression of hemolysis with an extended dosing interval.
Aims: ALXN1210-PNH-103 is a Phase 1/2, multicenter, open-label, intrapatient dose-escalation study (NCT02598583), evaluating the safety, tolerability, and efficacy of 2 IV maintenance dosing regimens of ALXN1210 in patients (pts) ≥18 y with PNH who were naïve to complement inhibitor therapy.
Methods: In this interim analysis, 6 pts in Cohort 1 (C1) received either 400- or 600-mg IV induction doses followed by a 900-mg maintenance dose q4w; 7 pts in Cohort 2 (C2) received 600- and 900-mg induction doses, followed by an 1800-mg maintenance dose q4w for up to 24 wk. The primary efficacy outcome was change in complement-mediated hemolysis as measured by LDH level. Other endpoints included changes in hematologic parameters, blood transfusions, FACIT-Fatigue scores, and pharmacokinetic (PK) parameters.
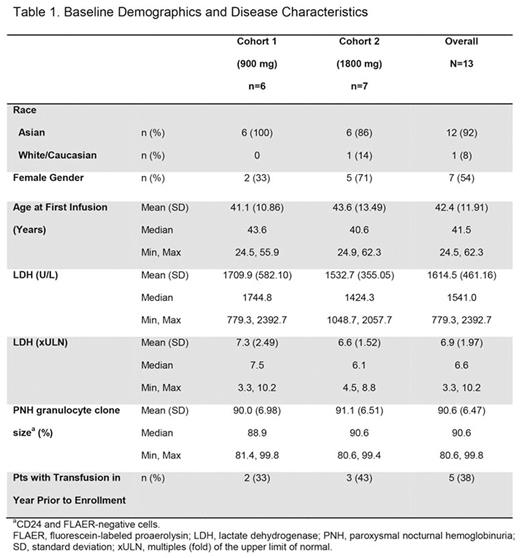
Results: A total of 13 pts consented and enrolled (Table 1). Median duration of exposure was 5.6 mo for C1 and 4.6 mo for C2. Pts had evidence of high hemolytic activity at baseline (BL), with LDH levels approximately 7-fold higher than the upper limit of normal (ULN). LDH levels decreased rapidly by the first evaluable time point (day 8), and improvements were sustained throughout all dosing intervals. Mean percentage reductions from BL in LDH levels were 85.9% in C1 at day 169 (n=6) and 85.2% in C2 at day 141 (n=5), the last available time points; respective LDH mean (SD) values at these time points were 232 (82) and 198 (36) U/L. Mean hemoglobin (Hb) levels were improved or stable in both cohorts. Among 5 pts with red blood cell transfusions in the 12 months prior to treatment (2 in C1, 3 in C2), only 1/2 pts (50%) in C1, became free of transfusions, while 3/3 pts (100%) in C2 were transfusion free. The pt in C1 who required transfusions had LDH levels of 391 and 417 U/L (1.67 and 1.78 x ULN), and Hb levels of 7.7 and 6.9 g/dL (BL Hb of 9.8 g/dL) on days 57 and 141, respectively. This pt consequently received 2 transfusions (2 units each) on these days. No pts in C2 required a transfusion.
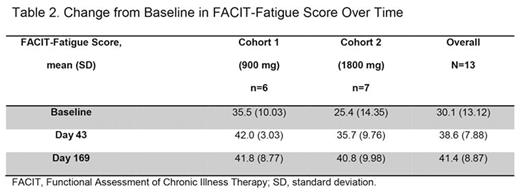
Mean (SD) FACIT-Fatigue scores increased from BL to day 43 by 28.9% (45.6%) in C1, and by 61.4% (49.9%) in C2 (Table 2). At day 169, FACIT-Fatigue scores were maintained in C1 (28.7% [52.7%] improvement from BL), but improved by 76.2% (70.3%) in C2. It should be noted that the BL FACIT-Fatigue score was lower in C2 than in C1 (10.1 points), indicating more severe fatigue.
PK analysis of available data revealed an estimated mean ± SD terminal (beta) half-life of ALXN1210 of 42 ± 6 days.
No deaths, serious adverse events (AEs), drug discontinuations, or AEs leading to withdrawals were reported in either cohort. The most common treatment-emergent AE (TEAE) was headache (4 pts). Investigators judged 77% of TEAEs to be unrelated to treatment. All AEs considered at least possibly related to therapy over 4.6 to 5.6 months of median exposure, including atrial flutter, general ache, headache, and worsening of anemia, resolved with ongoing ALXN1210 treatment.
Conclusion: In pts with PNH previously-naïve to complement inhibitor therapy, ALXN1210 treatment resulted in rapid, complete, and sustained C5 inhibition. LDH levels were rapidly reduced in all pts. Mean LDH levels were reduced to below the ULN in 4/6 (67%) of pts in C1, and in 6/7 (86%) of pts in C2 at the last evaluable time point. While there was a notable decrease in the need for blood transfusions, 17% (1/6) of pts in C1 (900 mg) experienced a recurrence of hemolysis and required transfusions, while no pts in C2 (1800 mg) had evidence of hemolysis or required a transfusion. FACIT-Fatigue scores improved in both cohorts, but improvement in patients was 2.1-fold greater in the higher dose cohort, consistent with better hemolytic response. These data suggest that the lower dose may be inadequate throughout the monthly dosing interval for complete suppression of hemolysis, which is central to the morbidities of PNH.
Supported by a model-derived preliminary mean ± SD terminal elimination half-life of 42 ± 6 days, a Phase 2 study examining longer dosing intervals of up to 12 weeks is ongoing.
Lee:Alexion Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Bachman:Alexion Pharmaceuticals, Inc.: Employment. Aguzzi:Alexion Pharmaceuticals, Inc.: Employment. Jang:Alexion Pharmaceuticals, Inc: Consultancy, Honoraria, Research Funding. Rottinghaus:Alexion Pharmaceuticals, Inc.: Employment. Shafner:Alexion Pharmaceuticals, Inc.: Employment. Szer:Ra Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alnylam: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal