Abstract
Background: The process known as shared decision-making (SDM) helps patients and providers make collaborative health care decisions that consider the available clinical evidence as well as the patient's values and preferences. Engaging the patient's participation in their own medical care is also a pillar of the National Strategy for Quality Improvement in Health Care. However, studies have shown a disconnect between provider perception of their ability to engage patients in their own care and patients' perception that their experiences and preferences are not being considered in the treatment decision-making process. Multiple Myeloma (MM) in particular is a complicated disease to manage with rapidly-evolving data and treatment standards. Treatment guidelines list multiple reasonable treatment options and often do not provide recommendations that are specific for individual patients. Therefore, patient-provider collaboration in treatment decision-making is a particularly important aspect of optimal MM care. This report aims to examine whether continuing medical education (CME) programming can help prepare physicians and other care providers to integrate the latest clinical data as well as the preferences of their patients into the decision-making process.
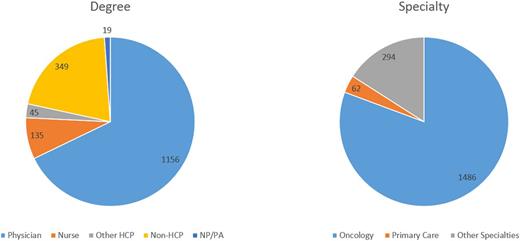
Methods: A CME-certified, video-based educational program was developed and posted on the Clinical Care Options' website on July 16, 2015. The educational program covered SDM concepts along with review of the clinical data and treatment guidelines critical to make optimal treatment decisions for patients with MM. Program participants were asked a series of 3 questions at the start of the program (baseline) and again after program completion (post-education). Data were collected from July 16, 2015 to December 4, 2015 from the 1253 program participants (64% MDs/NPs/PAs, 7% nurses; Figure 1) The impact of the education was calculated and reported as a Cohen's d effect using a matched-pair response comparison (n = 85) from the baseline and post-education questions.
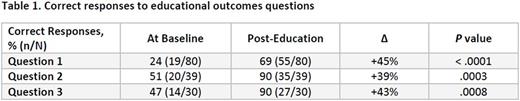
Results: At baseline, only 24% of program participants indicated that they would consider patient preferences as part of their decision-making process (Table 1). This significantly increased to 69% (45% improvement; P < .0001) among those that completed the program. The second question exposed program participants to a case vignette that challenged their ability to consider the available data along with patient preferences when making a treatment decision for a patient with MM. At baseline, only 51% of participants chose a treatment course that matched expert recommendations. However, after program completion, 90% chose the optimal answer, representing a 39% improvement (P = .0003). Finally, when asked to explain and manage a treatment-related adverse event, 47% of participants chose the optimal response at baseline and after completing the educational activity, 90% chose the optimal response, representing a 43% improvement (P = .0008). Overall, practical significance testing indicated that the activity had a positive impact on learners, with a Cohen's d effect size calculation of +1.41. In a post activity survey, each participant was asked how many of their patients were likely to benefit from their participation in this activity; the results totaled 7993 patients.
Conclusions: These data strongly suggest that CME programs focused on SDM can help prepare providers to consider patient preferences along with the available clinical data to make treatment decisions most likely to result in outcomes that matter most to their patients. Further randomized studies would be warranted to confirm these results. A full review of the study results and recommendations will be presented. This program was supported by independent educational grants from Celgene Corporation, Onyx Pharmaceuticals, and Takeda Pharmaceuticals North America, Inc.
Overall Learner Breakdown by Degree and Specialty
Kumar:Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Research Funding; BMS: Consultancy; Onyx: Consultancy, Research Funding; Kesios: Consultancy; Sanofi: Consultancy, Research Funding; AbbVie: Research Funding; Glycomimetics: Consultancy; Janssen: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal