Abstract
Myelodysplastic syndromes (MDS) are clonal stem cell diseases of the bone marrow (BM) characterized by a dysfunction of hematopoiesis commonly resulting in cytopenias, dysplasia and an increased risk of acute myeloid leukemia (AML) development. In up to 90% of MDS cases acquired somatic mutations can be identified. Additionally, these aberrations constitute important markers for diagnosis and risk stratification. Currently, the use of BM aspirates is the gold standard for cytogenetic and molecular genetic analysis. However, frequent analyses of peripheral blood (PB) samples are of special interest for monitoring the natural course and therapy response in MDS since this might not be feasible with BM specimens due to ethical and other reasons. The aim of this study was to investigate whether the high sensitivity of targeted deep sequencing (TDS) allows reliable detection of somatic variants also from PB samples and whether the molecular profiles are comparable between these different sources of material. Additionally robustness, feasibility and comparability of the method were verified by inter-laboratory comparisons.
This study included 31 patients from two centers, in Barcelona (Spain) and Göttingen (Germany) (12x RCMD, 4x RAEB-2, 4x sAML, 4x CMML-1, 2x MDS-RA, 1x MDS-U, 1x RAEB-1, 1x RCMD-SA, 1x RARS, 1x CCUS). For all patients, genomic DNA was extracted from concurrent mononuclear or total bone marrow cells (BMC), mononuclear peripheral blood cells (PBMC) and immunomagnetically (MACS) enriched circulating CD34+ cells (CD34+). Library preparation was performed using a custom hybridization-probe based panel (Nimblegen SeqCap EZ, Roche) including 83 myeloid-related genes (Barcelona) or the TruSight Myeloid Sequencing-Panel (Illumina) including 53 target genes (Göttingen). Sequencing was performed on MiSeq instruments. Reads were analyzed using local bioinformatic pipelines. Variants were filtered according to read depth (> 100x), population frequency (< 1%), their impact on protein integrity or function and evaluated by visualization on the Integrative Genome Viewer Software. Somatic origin of the variants was confirmed by sequencing of control samples from MACS enriched circulating T-lymphocytes (CD3+).
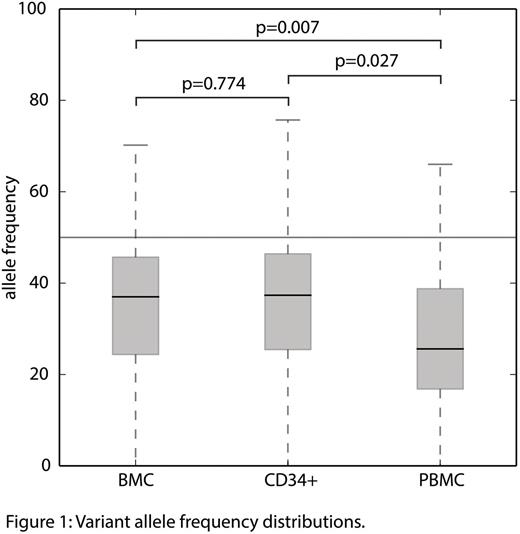
Somatic mutations were discovered in 29 of the 31 analyzed patients. Overall we identified 76 aberrations in 22 genes (12x TET2, 7x SF3B1, 7x SRSF2, 5x EZH2, 5x ASXL1, 5x U2AF1, 5x RUNX1, 4x ZRSR2, 4x TP53, 3x NRAS, 3x STAG2, 2x DNMT3A, 2x IDH1, 2x BCOR, 2x KRAS, 2x PTPN11, 1x PDGFRB, 1x IKZF1, 1x IDH2, 1x ATRX, 1x CSNK1A1, 1x ETV6). Literally all variants were found in BMC (n=74) as well as in circulating CD34+ cells (n=72) and PBMC (n=73). The discordance between the three sample types was random (5x RCMD, 2x sAML, 1x MDS-RA, 1x RARS). However, for five variants the allele frequency (VAF) values, which correlate with the clone size of the malignant cell population, were below 5% in PBMC samples. Therefore these variants were likely to be overlooked in case only PBMC would have been tested. There was no significant difference (p=0.774) between the VAF values measured in BMC (average: 40.0%) and enriched CD34+ cells (average: 41.3%). In contrast VAF values of PBMC (average: 30.1%) deviate significantly from both, BMC (p=0.007) as well as circulating CD34+ cells (p=0.027) (Figure 1).
Our findings indicate that TDS enables the adequate detection of somatic mutations from BM, circulating CD34+ cells and for the most part also in PBMC preparations. However, the malignant cell population seems to be less abundant in the PBMC fraction and therefore the detection and especially the quantification of clonal somatic variations is more challenging in this sample type. In the present study we demonstrate that enrichment of circulating CD34+ cells from PB can overcome this problem and provide data that are equivalent to the results obtained from the analysis of BMC, especially for follow up or minimal residual disease analyses.
We conclude that enrichment of CD34+ cells from PB constitutes an appropriate alternative for the reliable detection and quantification of somatic aberrations in MDS patients. Usage of circulating CD34+ cells in routine diagnostic next generation sequencing applications would significantly reduce invasive clinical interventions and could therefore improve diagnostics and disease monitoring.
Support: PI 11/02010, PI/14/00013, RD12/0036/0044, AR 14/34, R14/03, SGR225
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal