Abstract
Introduction: Hematopoietic cell transplantation (HCT) is a potentially curative treatment for patients with hematologic malignancies. Previous work using a national claims database identified the high cost of HCT, but was limited to the first 100 days. This study describes the rate of complications, medication use, and incremental total costs over a 2-year period among commercially insured patients who received autologous (auto-) or allogeneic (allo-) HCT in the US.
Methods: Patients with hematologic malignancies aged ≥18 years undergoing auto-HCT or allo-HCT (ICD-9 procedure codes 41.01-41.09) between January 1, 2011 and June 30, 2014 were identified in the Truven Health MarketScan Research Databases. Patients were required to have 12-month continuous enrollment pre- and post-HCT (index event), and those with prior HCT were excluded. Controls were selected from a pool of patients without HCT and matched in up to a 3:1 ratio on age, gender, insurance type, Deyo-Charlson comorbidity index, and diagnosis. Control patient index dates were randomly assigned based on the HCT index date distribution. Potential HCT-related complications were defined as having a medical claim with a diagnosis code for relapse, infection, cardiovascular, renal disease, neurological, pulmonary, hepatic, gastrointestinal, secondary malignancy, thrombotic microangiopathy or posterior reversible encephalopathy syndrome during the 12-month post index period. Total healthcare costs were defined as sum of health plan and patient paid costs for prescriptions and medical services, including inpatient admissions, emergency room and physician office visits, and other outpatient services. Incremental costs approach was used to examine the excess post-index all-cause costs of HCT by comparing auto-HCT or allo-HCT cohorts with corresponding controls.
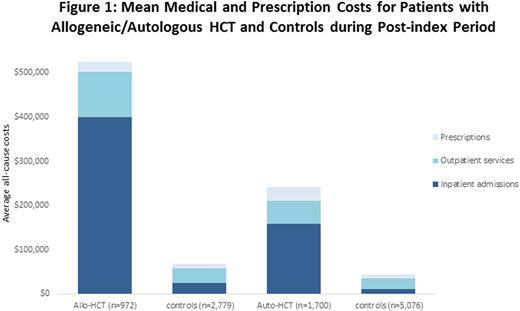
Results: 10,527 patients (HCT = 2,672; controls = 7,855) were included, with 95.7% of HCT recipients matched with 3 controls. The mean (± SD) age of HCT recipients was 54.5 (±11.6) years, 40.5% were female, and 63.6% underwent auto-HCT. Acute myeloid leukemia, myelodysplastic syndrome, myelofibrosis and myeloproliferative disease (69.1%) were the most common indications for allo-HCT, while lymphoma (39.5%) was the most common indication for auto-HCT. Compared with their controls, allo-HCT recipients had a significantly higher use of antivirals (91.5% vs 21.5%, p<0.01), antibiotics (91.0% vs 65.5%, p<0.01), and antifungals (85.3% vs 13.5%, p<0.01) during the post-index period. Patients in the allo-HCT cohort incurred $455,853 more total all-cause costs than their controls (p < 0.01, Figure 1), with 82.0% of the incremental difference due to inpatient admissions, 15.4% from outpatient services, and 2.6% from prescriptions. These differences in costs were mainly driven by longer hospital stays (23.2 vs 7.2 days, p<0.01). 95.5% of allo-HCT recipients had a complication that mostly occurred within 180 days post-HCT. Allo-HCT recipients with complications had higher mean annual post-index total costs compared to those without ($533,999 vs. $325,365). 88.7% of auto-HCT recipients used antibiotics and 32.7% used antifungals, which were all higher than those of controls (p<0.01). During the one-year post-index period, the incremental all-cause health care costs associated with auto-HCT were $198,687 (p< 0.01, Figure 1), of which 74.0% were due to inpatient admissions, 14.3% from outpatient services and 11.7% from prescriptions. Auto-HCT recipients also had significant longer hospital stays (18.2 vs 5.4 days, p<0.01) than controls. 81.0% of auto-HCT recipients had a complication after transplantation, and had higher annual post-index total costs than those without ($255,256 vs $185,395).
Conclusions: Hematologic malignancies are associated with considerable clinical and economic burdens. This analysis demonstrates that HCT adds significantly to the burden of therapy, with HCT recipients incurring about 10 times the cost of care during the 12-month post-transplantation period compared to controls. This analysis does not compare the long-term (e.g., ≥12-months) outcomes, and also underestimates total costs as it does not include indirect costs, caregiver burden, or donor costs. As expected, post-HCT complications further contribute to costs and this should encourage strategies that decrease complications and reduce costs.
Josephson:Seattle Genetics: Employment. Garfin:Seattle Genetics, Inc.: Employment. Richhariya:Seattle Genetics, Inc.: Employment. Bonafede:Truven Health: Other: I am an employee of Truven Health, which received a research contract to conduct this study with Seattle Genetics. . Donna:Truven Health: Other: I am an employee of Truven Health, which received a research contract to conduct this study with Seattle Genetics. . Cai:Truven Health Analytics: Other: I am an employee of Truven Health, which received a research contract to conduct this study with Seattle Genetics. , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal