Abstract
Background
In a Phase III COMPLEMENT 2 study,ofatumumab(OFA) plusfludarabine(F) and cyclophosphamide (C) demonstrated significantly improved median progression-free survival (PFS) by 54% compared to FC treatment alone (HR=0.67, p=0.0032) in patients with relapsed chronic lymphocytic leukemia (rCLL).
However, the relative value of OFA in rCLL has not been formally assessed. The objective of this study was to estimate the incremental cost per (quality-adjusted) life-year of utilizing OFA+FC vs. FC for rCLL in the US.
Methods
A partition survival model was developed to estimate the expected outcomes and costs of treatment of OFA+FC vs FC forrCLLover a lifetime horizon. The model includes 4 health states: PFS on treatment, PFS off-treatment, post-progression and death. Time during PFS following protocol-defined treatment duration of 6 months, was considered a treatment-free period in the model. Data on PFS, OS and frequencies of adverse events (AEs)were obtained from the Phase III clinical trial for OFA (COMPLEMENT 2). For the extrapolation of OS and PFS a piecewise approach was used, where the efficacy was based on the patient-level data (Kaplan-Meier Survivor Function) until the trial cut-off and a tail extrapolation thereafter (gamma distribution). Health state utilities and dis-utilities for AEs were obtained from previously published vignette studies. Costs incorporated in the model included drug and administration for primary and follow-up therapies, adverse event treatments, medical costs for hospitalizations and physician visits; and end of life costs. The costs were derived from databases (AnalySourceOnline, AHRQ, CMS).
Results
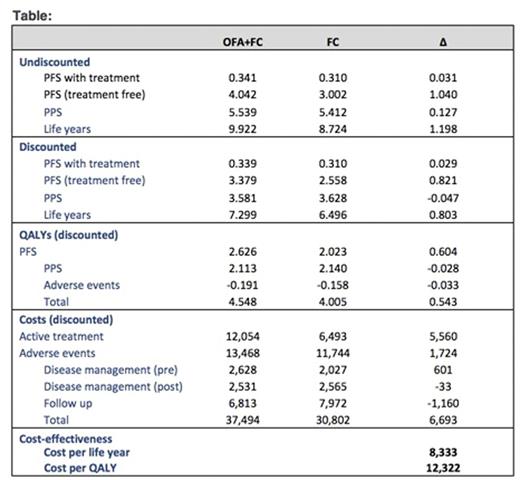
Treatment with OFA+FC led to an increase of 0.803 life years and 0.543 quality-adjusted life years (QALYs) relative to FC. The total cost of OFA+FC was higher by $6,693 per patient relative to FC. Although addition of AFA to FC lead to higher drug and adverse event costs, these were partially offset by lower follow-up costs compared to FC. The ICER per LY and per QALY gained with OFA+FC vs. FC was $8,333 and $12,322, respectively. Based on probabilistic sensitivity analyses, there wasa85% probability that OFA+FC was cost-effective compared to FC at a societal willingness-to-pay threshold of $100,000 per QALY saved.
Conclusions
Our analysis suggests treatment with OFA+FC compared to FC is highly cost-effective based Phase 3 within-trial analysis. These results are driven by the improved PFS and OS of OFA+FC vs. FC, as well as the treatment-free period, during which patients experienced PFS without the burden of treatment AEs or costs. Future direct comparisons of OFA+FC versus other treatment options will further clarify the cost-effectiveness of OFA+FC to inform coverage and reimbursement policy decisions.
Tremblay:Novartis Pharmaceuticals Corporation: Consultancy. Forsythe:Novartis Pharmaceuticals Corporation: Consultancy. Bal:Novartis Pharmaceuticals: Employment. Santra:Novartis Pharmaceuticals Corporation: Employment. Briggs:Novartis Pharmaceuticals Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal