Abstract
Background: Rituximab is an anti-CD20 monoclonal antibody commonly used in hematologic and oncologic conditions. Due to risk of infusion reaction, the first infusion is started at a slow rate with gradual increases as tolerated. Infusions take several hours, resulting in high cost, longer wait time, and decreased patient satisfaction. The risk of infusion reaction is highest with the first exposure (27-77% depending on indication). Patients who tolerate a first dose at the standard infusion rate have a markedly reduced risk of reaction with subsequent infusions (<10%). Rapid infusions of subsequent doses are well tolerated and standard practice in adults. The FDA approved a 90 minute infusion for adults with certain forms of lymphoma. Published data support a 120 minute infusion for adult rheumatology patients. Few data exist regarding the safety of rapid infusion of rituximab in children.
Objective: To determine the safety of rapid infusion of rituximab in children with hematologic, oncologic, and rheumatologic disorders; and to determine the incidence of infusion-related reactions.
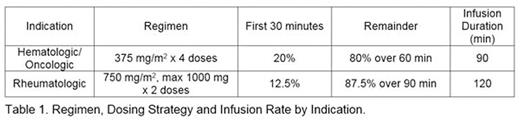
Methods: Patients receiving standard doses of rituximab for hematologic, oncologic or rheumatologic indications were eligible for enrollment in this IRB approved, single institution, prospective, single arm, open label pilot study. This study is registered at clinicaltrials.gov (NCT 02484053). Inclusion criteria were: age 1 to 20 years receiving outpatient rituximab infusion; at least one rituximab infusion via standard administration rate within the 28 days prior to study dose; and no history of grade 3 or 4 infusion reaction to rituximab, based on the NIH Common Terminology Criteria for Adverse Events version 4.03. Study patients received the same premedication regimen as they received with prior rituximab infusion(s); and included at minimum a single standard dose of acetaminophen and diphenhydramine prior to the start of the infusion. Corticosteroid premedication was used at physician discretion. Dosing and infusion rate depended on indication (Table 1).
The study was powered to demonstrate that the percentage of patients developing any dose-limiting toxicity (DLT) is ≤10%. Stopping rules were established utilizing the Bayesian optimal interval (BOIN) design. With a target enrollment of 20 patients, the number of DLT's expected was ≤1.7.
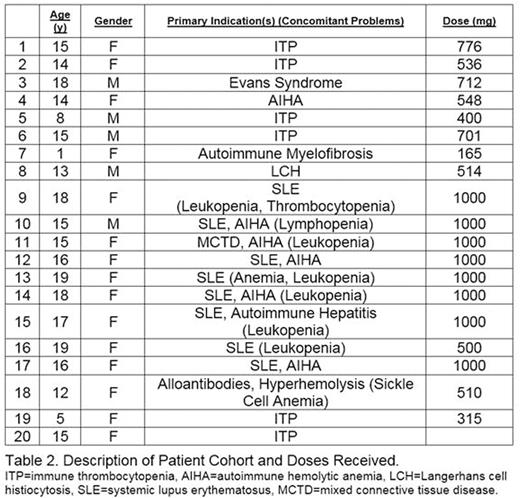
Results: Enrollment is complete with 20 enrolled and 19 evaluable patients. Patient demographics are provided (Table 2). 75% of patients were female; 60% were of Hispanic ethnicity. All patients tolerated the rapid infusion of rituximab and no patients had an infusion-related reaction. Two patients experienced expected rituximab related adverse events unrelated to the infusion rate, including muscle spasm and intermittent joint pain in 1 patient, and urticarial rash 1 week following infusion in 1 patient. There were no rate-limiting toxicities.
Conclusion: Rapid infusion of rituximab was well-tolerated in this cohort of pediatric patients treated with standard doses for hematologic, oncologic, and rheumatologic indications. There were no infusion reactions, consistent with adult data demonstrating safety of this approach. Additional benefits include decreased infusion duration, healthcare costs, and patient wait times, as well as improved patient satisfaction.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal