Abstract
Background: While reduced intensity conditioning has allowed older patients to undergo allogeneic hematopoietic stem cell transplantation (HSCT) for a variety of hematologic malignancies, age is still viewed as a relative contraindication. In particular, only limited and conflicting data are available on outcomes of patients ≥ 70 years old.
Methods: To determine outcomes and utility or futility of transplant for "older" patients, we conducted a retrospective study of all patients 65 and older who underwent allogeneic SCT at our institution between March 2002 and March 2015. 33 patients age 70-75 (median 71 years) were compared to 95 concurrent allogeneic HSCT recipients age 65-69 (median 67).
Results: The two groups (70-75 and 65-69) were similar in terms of donor type (70% URD 30% MRD vs. 67% URD 27% MRD 3% cord blood, p=0.86), conditioning regimen (75% flu/bu vs. 65% flu/bu, p =0.26), HCT-CI scores (mean 2.7 vs 2.9, p=0.29), disease phase (9% vs. 15% with primary refractory/active disease, p=0.651) and disease distribution (AML/MDS in 76% and 80%, p=0.7).
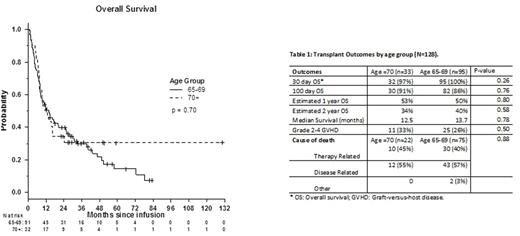
The OS for all patients was 51% at 1 year and 38% at 2 years, with a median follow up of 13.1 mo and no differences between age groups (fig 1, p=0.70). The median survival was 12.5 months in the 70-75 group vs. 13.7 months in the 65-69 group. As seen in Table 1, transplant was well tolerated and outcomes similar between both age groups with minimal 30 day mortality. Comparing patients age 70-75 to those 65-69, there was no difference in OS at 100d, 1 year or 2 years. There was no difference in grade II-IV acute GVHD (p=0.50). The cause of death was similar in both groups; of the patients who died, death was treatment-related in 45% and 40% of 70+ versus younger patients respectively, largely due to GVHD and infection. Relapse accounted for death in 55% and 57% of older versus younger patients who died.
Conclusions: These data suggest that at least selected patients ≥70 years old can tolerate allogeneic HSCT similar to patients ages 65-69. Outcomes in both age groups remain limited by a high risk of relapse highlighting that better methods for tumor control are needed such as post-transplant maintenance or novel disease-specific strategies that minimize GVHD but allow for enhanced GVL induction. However age ≥70 should not be an absolute contraindication for transplant.
Shaw:Vitality Institute: Research Funding; Novartis: Research Funding. Frey:Servier: Consultancy; Amgen: Consultancy; Novartis: Research Funding. Gill:Novartis: Patents & Royalties, Research Funding. Luger:Astellas: Honoraria; Dava Oncology: Honoraria; Pfizer: Consultancy; Onconova: Other: Clinical Trial --Institutional PI; Seattle Genetics: Other: Clinical Trial--Institutional PI; Ariad: Other: Clinical Trial--Institutional PI; Celgene: Other: Clinical Trial--Institutional PI; Cyclacel: Other: Clinical Trial, Institutional PI. . Mangan:Novartis: Research Funding; Incyte: Other: Advisory Board. Stadtmauer:Celgene: Consultancy; Takeda: Consultancy; Teva: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Amgen: Consultancy. Porter:Genentech: Other: Spouse Employment; Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal