Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is widely used in the treatment of a variety of hematologic diseases. However, allo-HSCT can be associated with many complications, including poor graft function early after transplant requiring long-lasting supportive care. In the literature, the incidence of poor graft function post allo-HSCT has been reported to range from 4 to 27%.
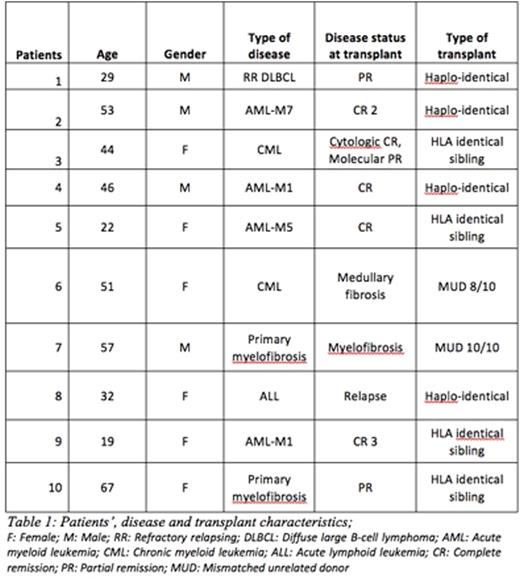
Here, we retrospectively studied 10 patients (male/female: 4/6, median age: 45 years, range 19 to 67) who received a boost of CD34+ selected cells for poor graft function after allo-HSCT (of whom 4 cases of haplo-identical allo-HSCT with post-Cy prophylaxis), between January 2014 and January 2016. Patients' disease and transplant characteristics are summarized in the below table.
Patients were selected for the CD34+ cells "boost" therapy after eliminating other causes that could explain a poor graft function (eg. drug toxicity, infections, disease relapse, etc.) The same original allo-HSCT donor was used to collect the CD34+ cells after mobilization with G-CSF and positive selection. The patients did not receive any prior conditioning therapy prior to CD34+ cells boost infusion. At time of the boost, all patients were in full donor chimerism. The number of infused CD34+ cells differed from one patient to another ranging from 2.91 to 7.99 x106 cells/kg recipient body weight. The median day of infusion post- allo-HSCT was 120 (range, 76-352). Among these 10 patients, 7 patients had full counts recovery at a median of 15 days (range, 7-30) post-infusion, while 3 patients had an incomplete response with persistent anemia and/or thrombocytopenia. None of the patients experienced clinically significant GVHD symptoms after the boost. At last follow-up, 7 patients were alive whereas 3 patients died of severe infections after 1, 6 and 13 months post-boost.
Based on these results, we concluded that boost therapy can be used in the treatment of poor graft function post-allogeneic HSCT, including in those patients who received a haplo-transplant.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal