Abstract
Introduction
Primary plasma cellleukemiais a rare and aggressive disorder. While autologous stem cell transplantation has been shown to improve outcome, response durations are short and further strategies are needed. Data is needed to help guide clinicians on the best approach to manage this disease. Therefore in an era of novel agents and improved autologous and allogeneic transplantation strategies this study compared different transplantation approaches in the treatment of PCL.
Material and Methods
A retrospective analysis was undertaken of theEuropean Group for Bone Marrow Transplantation (EBMT) experience of patients with primary PCL undergoing hematopoietic stem cell transplantation between 1998 and 2012. Only patients who had achieved complete response, partial response or stable disease prior to transplantation were included. Patients with progressive or relapsed disease were excluded.
Data was collected using MED A and MED B forms. The primary end point was overall survival and data was analysed according to the information on the planned transplant strategy reported at the time of first transplant (intent-to-treat principle).
Results
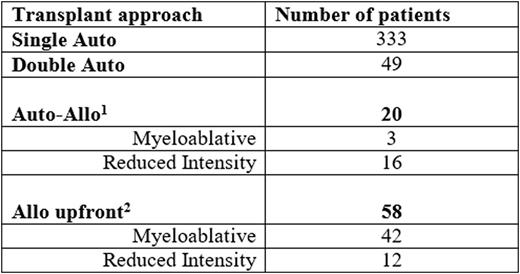
A total of 460 patients were identified and categorised into 4 transplant groups.
1. One patient did not proceed to Allo
2. Four patients not classified
The follow up period ranged from 1 to 208 months with a median follow up of 48.9 months.
Patients undergoing allo upfront were found to have the worst overall survival. Compared to single auto this was statistically significant with a Hazard Ratio (HR) 1.85 (p=0.007).
Compared to auto-allo the allo up front group had an even higher risk HR 3.18 (p=0.018). The double auto versus single auto had a HR 1.39 which was not statistically significant (p=0.28).
The auto-allo compared to single auto has a time varying effect with a higher mortality at the beginning and better risk afterwards. The effect on average is not significant HR 0.58 (p=0.24).
When we consider time varying effects three periods were examined, 0-12, 12-36 and 36-60 months. Allo upfront is still confirmed to be inferior to single auto and also to auto-allo in the first year. Auto-allo has a trend to superiority versus single or double auto in the mid-term. However due to lack of follow up data in the auto-allo group it is difficult to assess possible benefits of auto-allo in the long term as there are few patients still at risk. Our initial analysis does suggest an advantage.
Preliminary analysis suggests that the auto-allo group enjoy the best progression free survival. The superiority of auto-allo versus allo upfront may be attributed to the higher rates of non-relapse related mortality observed in allo upfront.
Conclusion
Our preliminary work has demonstrated that allo upfront is associated with the worst overall survival. Further data is needed to reliably assess the outcome of auto-allo group which appears to be superior. Therefore we plan to request additional information from involved centres to strengthen the power of our results.
Foà:Ariad: Speakers Bureau; Pfizer: Speakers Bureau; BMS: Consultancy; Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Janssen-Cilag: Consultancy, Speakers Bureau; Genetech: Consultancy; Roche: Consultancy, Speakers Bureau. Garderet:Takeda: Consultancy; Amgen: Consultancy; BMS: Consultancy, Honoraria; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal