Abstract

Introduction: The combined use of genetic markers and minimal residual disease (MRD) assessment identifies CLL patients (pts) with a poor outcome after front line chemoimmunotherapy. The CLLM1 study was initiated to evaluate lenalidomide as a maintenance treatment versus (vs) placebo in a double-blind fashion. The primary endpoint was progression free survival (PFS) by an independent review.
Methods: Pts who achieved at least a partial response after at least 4 cycles of front line chemoimmunotherapy were defined as high risk for progression if they had MRD levels of ≥10-2 or MRD levels of ≥10-4 to <10-2 combined with either an unmutated IGHV gene status, del(17p) or TP53 mutation at baseline. After 2:1 randomization, treatment with lenalidomide or placebo started with 5 mg daily in the first cycle, and was subsequently escalated to the target dose of 15 mg in the 7th cycle. Further possible escalation was guided by six-monthly MRD assessments. If well tolerated, the study drug was administered until disease progression. According to their risk for thromboembolic events pts received either low dose aspirin daily, or appropriate anti-coagulation prophylactic therapies. Pts were followed for progression monthly. This formal interim analysis was conducted after 20% (24 events) of the calculated PFS events and 89 pts of the planned 200 pts were randomized. PFS was evaluated at the significance level determined using the Hwang-Shih-DeCani spending function (including stopping boundaries for both futility and efficacy). The trial was registered with clinicaltrials.gov (NCT01556776).
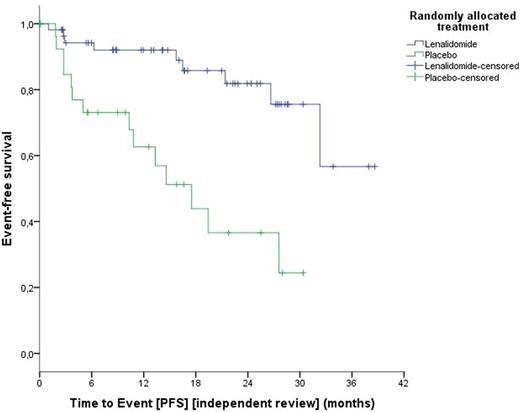
Results: A total of 468 pts from 62 sites in five countries (Austria, Germany, Italy, Netherlands and Spain) were screened for the study. 379 were not eligible, 347 because of MRD-negativity/low risk after frontline treatment and 32 because of progression between end of frontline and screening (11), withdrawal of consent (15) and other reasons (6). Out of 89 randomized pts, 60 received lenalidomide and 29 placebo. Randomized pts had a median age of 64 (range, 32-80), a median CIRS Score of 2 (range, 0-6), and 85.2% were male. 11.4% /20.5% of the pts had a 17p deletion/TP53 mutation, and 90.2% of the pts had an unmutated IGHV gene status at baseline. At randomization, 37% of pts had a high and 63% an intermediate MRD level, respectively. At data cut off, pts had received a median of 10 (range 0-42) treatment cycles [lenalidomide, 11 (0-42); placebo, 9 (1-35)]. Treatment was discontinued in 27 pts on lenalidomide and in 21 pts on the placebo arm due to adverse events (17 vs 6 pts), disease progression (4 vs 13 pts), withdrawal of consent (4 vs 2 pts) and other reasons (2 vs 2 pts). Treatment with lenalidomide was more frequently associated with neutropenia (30.4% vs 3.4%), gastrointestinal disorders (55.4% vs 27.6%), nervous system disorders (30.4% vs 13.8%), respiratory disorders (35.7% vs 13.8%) and skin disorders (60.7% vs 27.6%). Infections (50% vs 62.1%) and vascular disorders (14.3% vs 17.2%) were not increased in the lenalidomide compared to the placebo arm. Three deaths have been observed so far, one in the lenalidomide arm (acute lymphoblastic leukemia) and two in the placebo arm (1 progressive multifocal leukoencephalopathy; 1 Richter's syndrome). After a median observation time of 17.7 months, the hazard ratio for PFS was 0.198 with 95% confidence interval (CI) 0.083 to 0.475 (stratified by MRD level at randomization). The median PFS in the placebo arm was 14.6 months, and was not reached in the lenalidomide arm (see figure 1). The one-sided p-value from the stratified log-rank test was 0.000059/2, thus smaller than the determined efficacy boundary 0.0006 derived from the Hwang-Shih-DeCani spending function based on 24 PFS events. Conversion to MRD negativity has been observed only in the lenalidomide arm, data will be presented at the meeting.
Conclusion: Lenalidomide is a feasible and efficacious maintenance option for high risk CLL after chemoimmunotherapy and substantially prolonged PFS in high risk CLL patients resulting in a relative risk reduction for progression of more than 80%. An independent data monitoring committee assessed the results as being robust and reliable and recommended unblinding of the study as well as continuing treatment with lenalidomide. The PFS observed in the placebo arm independently confirms the prognostic significance of the MRD based risk assessment model, which might be used in future trials.
Fink:Mundipharma: Other: Travel grants; Roche: Honoraria, Other: Travel grants; Celgene: Research Funding; AbbVie: Other: Travel grants. Bahlo:Roche: Honoraria, Other: Travel grants. Al-Sawaf:Gilead: Other: Travel grants. Fischer:Roche: Other: travel grants. Wendtner:Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffmann-La Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ghia:Janssen: Consultancy, Honoraria, Speakers Bureau; Roche: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Adaptive Biotechnology: Consultancy; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau. Bosch:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kater:Celgene: Research Funding. Kreuzer:Gilead Sciences: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche Pharma GmbH and Mundipharma GmbH: Consultancy, Honoraria, Research Funding, Speakers Bureau. Kneba:AbbVie: Consultancy, Honoraria, Other: Travel grants; Janssen-Cilag: Consultancy, Honoraria, Other: Travel grants; Roche: Consultancy, Honoraria, Other: Travel grants, Research Funding; Amgen: Research Funding; Gilead: Consultancy, Honoraria, Other: Travel grants, Research Funding; Glaxo-SmithKline: Other: Travel grants. Stilgenbauer:Gilead: Consultancy, Honoraria, Other: Travel grants , Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel grants , Research Funding; GSK: Consultancy, Honoraria, Other: Travel grants , Research Funding; Janssen: Consultancy, Honoraria, Other: Travel grants , Research Funding; Novartis: Consultancy, Honoraria, Other: Travel grants , Research Funding; Amgen: Consultancy, Honoraria, Other: Travel grants, Research Funding; Mundipharma: Consultancy, Honoraria, Other: Travel grants , Research Funding; Sanofi: Consultancy, Honoraria, Other: Travel grants , Research Funding; Boehringer Ingelheim: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genentech: Consultancy, Honoraria, Other: Travel grants , Research Funding; Celgene: Consultancy, Honoraria, Other: Travel grants , Research Funding; Hoffmann-La Roche: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genzyme: Consultancy, Honoraria, Other: Travel grants , Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel grants, Research Funding. Boettcher:Celgene: Research Funding. Eichhorst:Mundipharma: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy; Roche: Consultancy, Research Funding, Speakers Bureau. Hallek:AbbVIe: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Glaxo-SmithKline: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; Celgene: Consultancy, Honoraria; Roche: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal