Abstract
Allogeneic stem cell transplantation (allo-SCT) offers a potentially curative option for eligible patients with poor-risk myeloid malignancies. The prognostic impact of specific mutations such as TP53 is unclear in this context1,2. We report the prognostic impact of mutations in a panel of 19 genes (covering entire coding regions of DNMT3A, CEBPA, GATA2, TET2, TP53 and mutation hot spots of ASXL1, BRAF, CBL, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, NPM1, NRAS, PTPN11, RUNX1 and WT1) identified by targeted sequencing in patients undergoing allo-SCT with FLAMSA-Bu conditioning.
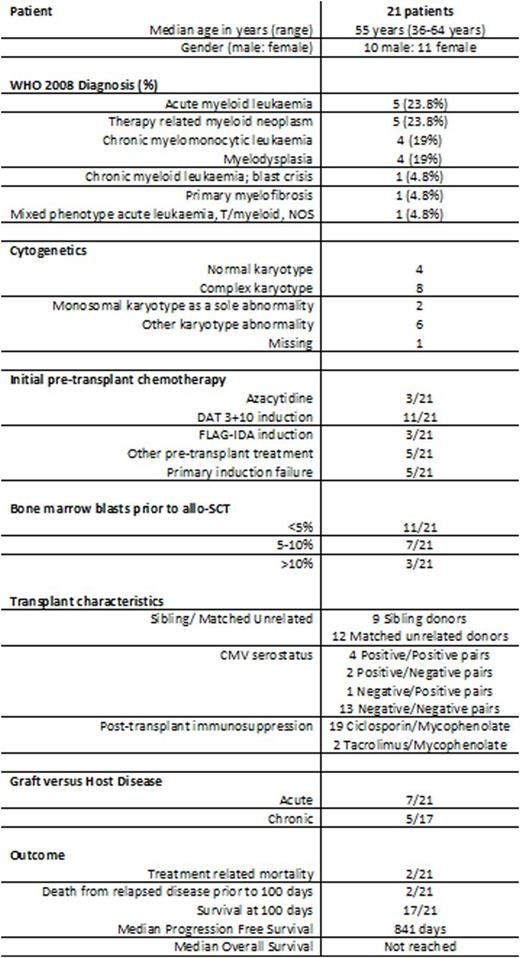
Twenty-one patients (10 male, 11 female; median age 55 years; range 36-64 years) were included and identified as having poor risk disease on the basis of acute myeloid leukemia (AML) with primary induction failure (n=5), myelodysplasia (MDS) with high or very high risk R-IPSS scores (n=8, 4 of whom had therapy related MDS), therapy related acute myeloid leukemia with MLL rearrangement (n=1), intermediate-2 or high risk prognostic score for chronic myelomonocytic leukemia (CMML) (n=4), blast crisis of chronic myeloid leukemia (n=1), primary myelofibrosis with blasts > 10% on bone marrow trephine (n=1), mixed phenotype acute leukemia (T/myeloid) with complex karyotype (n=1). Overall, complex karyotypes were detected in 8/21 (38.1%) patients. The median Hematopoietic Cell Transplantation-Comorbidity Index score was 4 (range 0-10).
All 21 patients underwent allo-SCT with fludarabine, cytarabine, amsacrine, busulphan, and anti-thymocyte globulin (FLAMSA-Bu) conditioning. Twelve (57.1%) received stem cells from fully HLA matched unrelated donors. Neutrophil engraftment occurred at a median of 24 days (range 11-124 days) and platelet engraftment at a median of 26 days (range 10-221 days) post-transplant. Seven (33.3%) patients developed acute graft versus host disease (GVHD). Ten (47.6%) patients received planned donor lymphocyte infusions.
Genomic DNA was available from 16/21 patient samples and was sequenced using the Ion-Torrent platform. Somatic driver mutations were identified in 13/16 (81.2%) patients, 10 of whom had two or more driver mutations. TET2 mutations were the most common lesion, detected in 6/16 (37.5%) cases, followed by RUNX1, ASXL1 and DNMT3A in 3/16 (18.8%) patients each.
Ten (47.6%) patients remain alive and disease-free after a median of 19.3 months follow-up. Two treatment-related deaths occurred; one from sepsis in the context of steroid-refractory GVHD and a second patient died of toxoplasmosis infection. Nine (42.9%) patients have relapsed post allo-SCT, three of whom remain alive following salvage therapy. The median progression free survival (PFS) is 841 days and the median overall survival (OS) has not yet been reached. Patients with therapy-related myeloid neoplasms trended towards shorter PFS and OS compared with all other diagnosis (396 vs 841 days, p=0.54; 373 days vs undefined, p=0.11, respectively). All four CMML patients have relapsed at a median of 694 days post FLAMSA-Bu allo-SCT. The median PFS for de novo AML and MDS has not been reached.
Monosomal karyotype was associated with a non-significant trend towards shortened PFS (148 days vs 751 days, p=0.11). Cases with TET2 mutations trended towards a shorter PFS compared with wild-type TET2 (751 days vs undefined, p=0.6407) but this did not reach statistical significance. No difference was observed in PFS between TP53 mutated vs wild-type TP53 cases (517 days vs 751 days, p=0.99).
FLAMSA-Bu allo-SCT remains a viable treatment option for selected patients with de novo AML and MDS, even patients in whom multiple or adverse somatic mutations are detected. Conversely, durable remissions are uncommon for patients with therapy-related myeloid neoplasms or CMML. These patients may benefit from consideration for alternative treatment strategies.
References:
1. Christopeit, M., Badbaran, A., Alawi, M., et al. (2016), Correlation of somatic mutations with outcome after FLAMSA-busulfan sequential conditioning and allogeneic stem cell transplantation in patients with myelodysplastic syndromes. Eur J Haematol. doi:10.1111/ejh.12724
2. Bejar, R., Stevenson, K.E., Caughey, B., et al (2014), Somatic Mutations Predict Poor Outcome in Patients With Myelodysplastic Syndrome After Hematopoietic Stem-Cell Transplantation. JCO 32(25) 2691-2698.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal